Improvement of MASLD and MASH by suppression of hepatic N-acetyltransferase 10
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
Metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) are characterized by excessive triglyceride accumulation in the liver. However, due to an incomplete understanding of its pathogenesis, more efforts are needed to identify specific and effective treatments. N4-acetylcytidine (ac4C) is a newly discovered RNA modification to regulate mRNA. N-acetyltransferase 10 (NAT10) has not been fully explored in MASLD and MASH.
Methods
The clinical relevance of NAT10 was evaluated based on its expression in various mouse and human models of MASLD and MASH. Acetylated RNA immunoprecipitation sequencing and mRNA stability assays were used to explore the role of NAT10 in regulating ac4C modification and expression of target genes. Genetically engineered mice were employed to investigate the role of NAT10 in MASLD and MASH progression.
Results
Hepatic NAT10 expression was significantly increased in multiple mice and humans of MASLD and MASH. Genetic knockout of NAT10 protected mice from diet-induced hepatic steatosis and steatohepatitis, whereas overexpression of NAT10 exacerbated high-fat-diet-induced liver steatosis. Mechanistically, NAT10 binds to Srebp-1c mRNA, promoting its stability and expression, thereby upregulating lipogenic enzymes. Treatment with Remodelin, a NAT10-specific inhibitor, effectively ameliorates liver steatosis and dyslipidemia in a preclinical mouse model.
Conclusions
Our findings indicate that NAT10 could regulate lipid metabolism in MASLD and MASH by stabilizing Srebp-1c mRNA and upregulating lipogenic enzymes. This study highlights the role of NAT10 and RNA acetylation in the pathogenesis of MASLD and MASH. Thus, our findings suggest a promising new therapeutic approach, such as the use of NAT10 inhibitor, for treating metabolic liver disease.
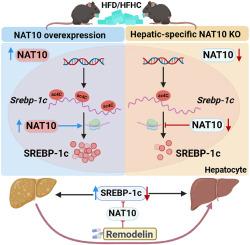
通过抑制肝脏 N-乙酰转移酶 10 改善 MASLD 和 MASH 症状
代谢功能障碍相关性脂肪性肝病(MASLD)和代谢功能障碍相关性脂肪性肝炎(MASH)的特点是肝脏中甘油三酯过度积累。然而,由于对其发病机理的了解尚不全面,因此需要做出更多努力来确定具体而有效的治疗方法。N4-乙酰胞苷(ac4C)是一种新发现的调节 mRNA 的 RNA 修饰。N-乙酰转移酶10(NAT10)在MASLD和MASH中的作用尚未得到充分探讨。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


