Development of Pro-resolving and Pro-efferocytic Nanoparticles for Atherosclerosis Therapy
引用次数: 0
Abstract
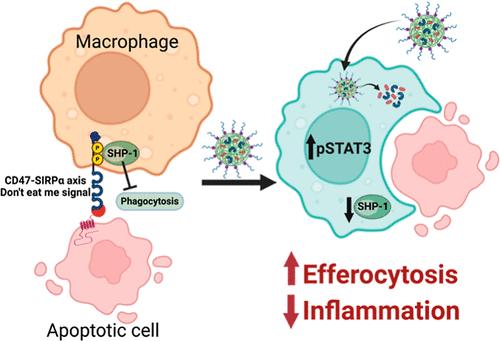
Atherosclerosis is a major contributor to cardiovascular diseases with a high global prevalence. It is characterized by the formation of lipid-laden plaques in the arteries, which eventually lead to plaque rupture and thrombosis. While the current lipid-lowering therapies are generally effective in lowering the risk of cardiovascular events, they do not address the underlying causes of disease. Defective resolution of inflammation and impaired efferocytosis are the main driving forces of atherosclerosis. Macrophages recognize cells for clearance by the expression of “eat me” and “do not eat me” signals, including the CD47-SIRPα axis. However, the “do not eat me” signal CD47 is overexpressed in atherosclerotic plaques, leading to compromised efferocytosis and secondary necrosis. In this context, prophagocytic antibodies have been explored to stimulate the clearance of apoptotic cells, but they are nonspecific and impact healthy tissues. In macrophages, downstream of signal regulatory protein α, lie protein tyrosine phosphatases, SHP 1/2, which can serve as effective targets for selectively phagocytosing apoptotic cells. While increasing the efferocytosis targets the end stages of lesion development, the underlying issue of inflammation still persists. Simultaneously increasing efferocytosis and reducing inflammation can be effective therapeutic strategies for managing atherosclerosis. For instance, IL-10 is a key anti-inflammatory mediator that enhances efferocytosis via phosphoSTAT3 (pSTAT3) activation. In this study, we developed a combination nanotherapy by encapsulating an SHP-1 inhibitor (NSC 87877) and IL-10 in a single nanoparticle platform [(S + IL)-NPs] to enhance efferocytosis and inflammation resolution. Our studies suggest that (S + IL)-NPs successfully encapsulated both agents, entered the macrophages, and delivered the agents into intracellular compartments. Additionally, (S + IL)-NPs decreased inflammation by suppressing pro-inflammatory markers and enhancing anti-inflammatory mediators. They also exhibited the potential for improved phagocytic activity via pSTAT3 activation. Our nanomedicine-mediated upregulation of the anti-inflammatory and efferocytic responses in macrophages shows promise for the treatment of atherosclerosis.
开发用于动脉粥样硬化治疗的促溶解和促吞噬纳米粒子
动脉粥样硬化是心血管疾病的主要诱因,在全球发病率很高。动脉粥样硬化的特点是动脉中形成富含脂质的斑块,最终导致斑块破裂和血栓形成。虽然目前的降脂疗法在降低心血管事件风险方面普遍有效,但它们并没有解决疾病的根本原因。炎症解决不力和渗出功能受损是动脉粥样硬化的主要驱动力。巨噬细胞通过表达 "吃我 "和 "不吃我 "信号(包括 CD47-SIRPα 轴)来识别需要清除的细胞。然而,"不要吃我 "信号 CD47 在动脉粥样硬化斑块中过度表达,导致细胞清除能力受损和继发性坏死。在这种情况下,人们探索了促吞噬抗体来刺激凋亡细胞的清除,但这些抗体是非特异性的,会影响健康组织。在巨噬细胞中,信号调节蛋白α的下游是蛋白酪氨酸磷酸酶SHP 1/2,它们可以作为选择性吞噬凋亡细胞的有效靶点。虽然增加流出细胞的数量可以针对病变发展的末期阶段,但炎症的根本问题仍然存在。同时增加细胞外排和减少炎症可以成为控制动脉粥样硬化的有效治疗策略。例如,IL-10 是一种关键的抗炎介质,它能通过磷酸化 STAT3(pSTAT3)的激活来增强流出细胞的功能。在本研究中,我们开发了一种联合纳米疗法,将 SHP-1 抑制剂(NSC 87877)和 IL-10 包封在一个纳米粒子平台[(S + IL)-NPs]中,以增强流出细胞和炎症消退。我们的研究表明,(S + IL)-NPs 成功地封装了这两种药物,进入巨噬细胞,并将药物输送到细胞内。此外,(S + IL)-NPs 还通过抑制促炎标志物和增强抗炎介质来减少炎症。它们还显示出通过激活 pSTAT3 提高吞噬活性的潜力。我们的纳米药物介导的巨噬细胞抗炎和吞噬反应的上调为治疗动脉粥样硬化带来了希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


