Translational modelling to predict human pharmacokinetics and pharmacodynamics of a Bruton's tyrosine kinase‐targeted protein degrader BGB‐16673
IF 6.8
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
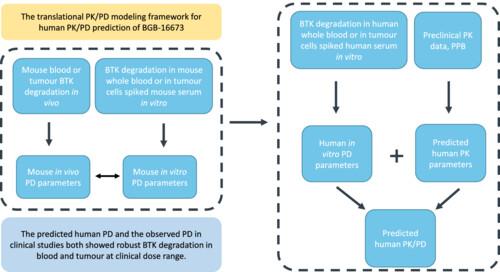
Background and PurposeBifunctional small molecule degraders, which link the target protein with E3 ubiquitin ligase, could lead to the efficient degradation of the target protein. BGB‐16673 is a Bruton's tyrosine kinase (BTK) degrader. A translational PK/PD modelling approach was used to predict the human BTK degradation of BGB‐16673 from preclinical in vitro and in vivo data.Experimental ApproachA simplified mechanistic PK/PD model was used to establish the correlation between the in vitro and in vivo BTK degradation by BGB‐16673 in a mouse model. Human and mouse species differences were compared using the parameters generated from in vitro human or mouse blood, and human or mouse serum spiked TMD‐8 cells. Human PD was then predicted using the simplified mechanistic PK/PD model.Key ResultsBGB‐16673 showed potent BTK degradation in mouse whole blood, human whole blood, and TMD‐8 tumour cells in vitro. Furthermore, BGB‐16673 showed BTK degradation in a murine TMD‐8 xenograft model in vivo. The PK/PD model predicted human PD and the observed BTK degradation in clinical studies both showed robust BTK degradation in blood and tumour at clinical dose range.Conclusion and ImplicationsThe presented simplified mechanistic model with reduced number of model parameters is practically easier to be applied to research projects compared with the full mechanistic model. It can be used as a tool to better understand the PK/PD behaviour for targeted protein degraders and increase the confidence when moving to the clinical stage.
通过转化建模预测布鲁顿酪氨酸激酶靶向蛋白降解剂 BGB-16673 的人体药代动力学和药效学
背景和目的将目标蛋白与E3泛素连接酶连接起来的双功能小分子降解剂可导致目标蛋白的有效降解。BGB-16673是一种布鲁顿酪氨酸激酶(BTK)降解剂。实验方法采用简化的机理PK/PD模型,在小鼠模型中建立BGB-16673对BTK的体外和体内降解之间的相关性。利用从体外人或小鼠血液以及人或小鼠血清中添加的 TMD-8 细胞中生成的参数,比较了人和小鼠的物种差异。主要结果BGB-16673在小鼠全血、人全血和体外TMD-8肿瘤细胞中显示出强效的BTK降解作用。此外,BGB-16673 在小鼠 TMD-8 异种移植模型体内也显示出 BTK 降解作用。PK/PD模型预测的人体PD和临床研究中观察到的BTK降解均表明,在临床剂量范围内,血液和肿瘤中的BTK降解十分强劲。它可以作为一种工具,用于更好地理解靶向蛋白降解剂的 PK/PD 行为,并在进入临床阶段时增强信心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.40
自引率
12.30%
发文量
270
审稿时长
2.0 months
期刊介绍:
The British Journal of Pharmacology (BJP) is a biomedical science journal offering comprehensive international coverage of experimental and translational pharmacology. It publishes original research, authoritative reviews, mini reviews, systematic reviews, meta-analyses, databases, letters to the Editor, and commentaries.
Review articles, databases, systematic reviews, and meta-analyses are typically commissioned, but unsolicited contributions are also considered, either as standalone papers or part of themed issues.
In addition to basic science research, BJP features translational pharmacology research, including proof-of-concept and early mechanistic studies in humans. While it generally does not publish first-in-man phase I studies or phase IIb, III, or IV studies, exceptions may be made under certain circumstances, particularly if results are combined with preclinical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


