Arylation of 2-Chloro-3-(4,6-Diaryl-1,3,5-Triazin-2-yl) Quinolines: Formal Synthesis of 3-(4,6-Diaryl-1,3,5-Triazin-2-yl)-2-Substituted Quinolines by Suzuki–Miyaura Reaction
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We have described herein a simple and formal two-step synthesis of 3-(4,6-diaryl-1,3,5-triazin-2-yl)-2-aryl quinolines from 2-chloro-3-(4,6-diaryl-1,3,5-triazin-2-yl) quinolines and boronic acids under the Suzuki–Miyaura conditions. This protocol provides the C-2 arylation of 2-chloro-3-(4,6-diaryl-1,3,5-triazin-2-yl) quinolines under the mild reaction conditions. These newly formed chemo-types may be useful in drug discovery programs or in material chemistry.
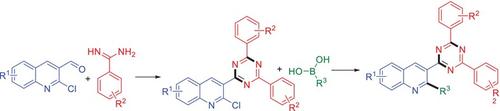
2-氯-3-(4,6-二芳基-1,3,5-三嗪-2-基)喹啉的芳基化反应:通过铃木-宫浦反应正式合成 3-(4,6-二芳基-1,3,5-三嗪-2-基)-2-取代的喹啉类化合物
我们在此介绍了一种简单而正规的两步合成法,即在铃木-宫拉条件下,由 2-氯-3-(4,6-二芳基-1,3,5-三嗪-2-基)喹啉和硼酸合成 3-(4,6-二芳基-1,3,5-三嗪-2-基)-2-芳基喹啉。该方案可在温和的反应条件下对 2-氯-3-(4,6-二芳基-1,3,5-三嗪-2-基)喹啉进行 C-2 芳基化反应。这些新形成的化学类型可能有助于药物发现计划或材料化学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


