Regulating the Electrochemical Performance of A2Ni2TeO6 (A = Na, K) as a Cathode of Alkali Metal Ion Battery by 3d Transition Metal Substitution from a Theoretical Perspective
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
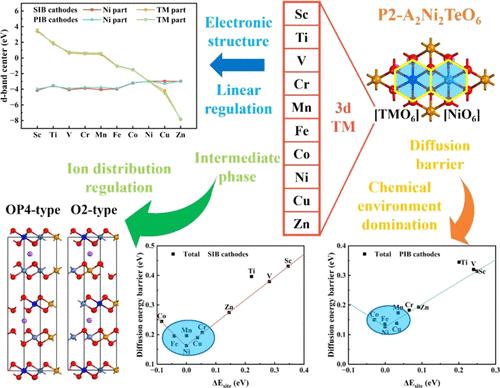
With its unique honeycomb layered structure, P2-type A2Ni2TeO6 (A = Na, K) exhibits remarkable cycling stability and ionic diffusion capability, making it a promising cathode material for sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs). However, the high operating voltage of A2Ni2TeO6 (A = Na, K) leading to surface degradation and CEI formation limits the capacity of A2Ni2TeO6 (A = Na, K), where only 2/3 of Na+ and 1/2 K+ can be reversibly extracted at a high charge rate. To enhance the capacity of A2Ni2TeO6 (A = Na, K) while maintaining its cycling stability, we delved into the impacts of 3d transition metal substitution on sodium and potassium storage chemistry through first-principles calculations. Our investigation includes multiple facets: lattice structure, substituting formation energy, electronic properties, ionic diffusion, average open-circuit voltage, transition metal migration, and intermediate phases in the high-voltage region. After comprehensive consideration, Mn- and Fe-substituted Na2Ni2TeO6 and Fe-substituted K2Ni2TeO6 emerged as the most promising candidates, exhibiting exceptional electrochemical performance. Furthermore, we discovered that the energy difference between alkali metal ions occupying substitution sites and active transition metal sites dominates the ionic diffusion behavior in TM-substituted A2Ni2TeO6 (A = Na, K), and the nonuniform distribution of alkali metal ions significantly contributes to the large volume change during ionic extraction. The findings of this work not only underscore the intricate structure–activity relationship of P2-type A2Ni2TeO6 (A = Na, K) substitution but also provide theoretical insights for future application of honeycomb layered transition metal oxides (HLOs) in SIB and PIB cathodes.
从理论角度看通过 3d 过渡金属替代调节 A2Ni2TeO6(A = Na、K)作为碱金属离子电池阴极的电化学性能
P2 型 A2Ni2TeO6(A = Na,K)具有独特的蜂窝状层状结构,显示出卓越的循环稳定性和离子扩散能力,使其成为钠离子电池(SIB)和钾离子电池(PIB)的一种前景广阔的阴极材料。然而,A2Ni2TeO6(A = Na,K)的高工作电压会导致表面降解和 CEI 的形成,从而限制了 A2Ni2TeO6(A = Na,K)的容量。为了在保持循环稳定性的同时提高 A2Ni2TeO6(A = Na、K)的容量,我们通过第一性原理计算深入研究了 3d 过渡金属取代对钠和钾存储化学性质的影响。我们的研究包括多个方面:晶格结构、替代形成能、电子特性、离子扩散、平均开路电压、过渡金属迁移以及高压区的中间相。经过综合考虑,锰和铁取代的 Na2Ni2TeO6 和铁取代的 K2Ni2TeO6 成为最有前途的候选物质,表现出优异的电化学性能。此外,我们还发现,在 TM 取代的 A2Ni2TeO6(A = Na、K)中,占据取代位点的碱金属离子与活性过渡金属位点之间的能量差主导了离子扩散行为,而碱金属离子的不均匀分布在很大程度上导致了离子萃取过程中的巨大体积变化。这项工作的发现不仅强调了 P2 型 A2Ni2TeO6(A = Na、K)替代物错综复杂的结构-活性关系,还为蜂窝层状过渡金属氧化物(HLOs)未来在 SIB 和 PIB 阴极中的应用提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


