Fluorobenzene-Diluted Localized Highly Concentrated Electrolyte for Enhanced Electrochemical Performance of Li–O2 Battery
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
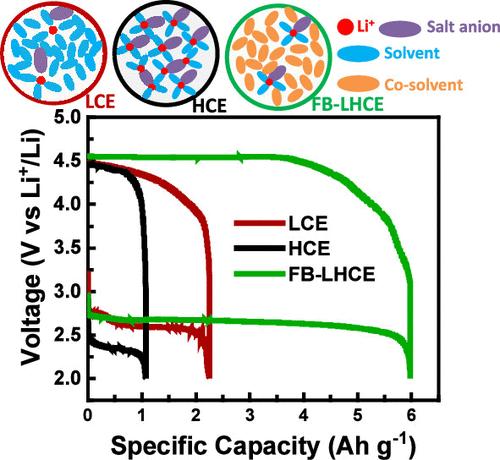
Highly concentrated electrolytes (HCEs), owing to their high thermal and chemical stability, wider electrochemical stability windows (ESWs), and enhanced stability with Li metal anode, have been under the spotlight as a potential electrolyte candidate for developing Li–O2 batteries. Nonetheless, their high viscosity, poor wettability, and high cost pose great challenges in achieving the desired results. In this study, we designed an HCE diluted with a low-polarity hydrocarbon cosolvent, fluorobenzene, and investigated its electrochemical performance in a Li–O2 battery. Raman spectroscopy analysis and self-diffusivity coefficients of electrolyte components determined by nuclear magnetic resonance (NMR) technique have confirmed that the fluorobenzene-based localized highly concentrated electrolyte (FB-LHCE) conserved the unique solvation structure of contact ion pairs and cation–anion aggregates formed in HCE. Incorporating fluorobenzene into HCE improved the self-diffusivity of electrolyte components by over a magnitude, lowered the viscosity by over 40 times, and increased the ionic conductivity 5-fold. Furthermore, FB-LHCE drastically improved the electrode wettability by yielding 3 and 5 orders of magnitude higher double-layer capacitances than those of low-concentration (LCE) and HCE, respectively. Additionally, when compared with LCE as the baseline, HCE and FB-LHCE have demonstrated wider anodic ESWs (>4.6 V). Li||Li symmetric cell tests revealed significantly improved electrochemical stability of HCE and FB-LHCE with Li metal anode compared to LCE. HCE and FB-LHCE have yielded stable cycling for double the amount of time (1300 h) compared with LCE (650 h). FB-LHCE delivered the highest specific discharge capacity (6.0 Ah g–1) followed by LCE (2.25 Ah g–1) and HCE (1.07 Ah g–1) in Li–O2 cells. Full-cell cycling stability tests have shown enhanced cycling stability with FB-LHCE (11 cycles) compared with those with LCE (2 cycles) and HCE (2 cycles).
氟苯稀释局部高浓度电解液用于增强二氧化锰锂电池的电化学性能
高浓度电解质(HCEs)具有较高的热稳定性和化学稳定性、较宽的电化学稳定窗口(ESWs)以及与锂金属阳极的稳定性增强等特点,一直是开发锂-O2 电池的潜在候选电解质。然而,它们的高粘度、低浸润性和高成本给实现预期结果带来了巨大挑战。在本研究中,我们设计了一种用低极性烃类共溶剂氟苯稀释的 HCE,并研究了它在锂-O2 电池中的电化学性能。拉曼光谱分析和核磁共振(NMR)技术测定的电解质成分自扩散系数证实,氟苯基局部高浓度电解质(FB-LHCE)保留了 HCE 中形成的接触离子对和阳离子-阴离子聚集体的独特溶解结构。在 HCE 中加入氟苯可将电解质成分的自扩散率提高一个量级以上,将粘度降低 40 倍以上,并将离子电导率提高 5 倍。此外,FB-LHCE 还大大改善了电极润湿性,其双层电容分别比低浓度(LCE)和 HCE 高出 3 个和 5 个数量级。此外,与作为基线的 LCE 相比,HCE 和 FB-LHCE 显示出更宽的阳极 ESW(4.6 V)。锂||锂对称电池测试表明,与 LCE 相比,HCE 和 FB-LHCE 与锂金属阳极的电化学稳定性明显提高。与 LCE(650 小时)相比,HCE 和 FB-LHCE 的稳定循环时间延长了一倍(1300 小时)。在锂-氧化物电池中,FB-LHCE 的比放电容量最高(6.0 Ah g-1),其次是 LCE(2.25 Ah g-1)和 HCE(1.07 Ah g-1)。全电池循环稳定性测试表明,与 LCE(2 次循环)和 HCE(2 次循环)相比,FB-LHCE(11 次循环)具有更高的循环稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


