Molecular Design Strategy toward Multielectron-Based Polyphenylaniline Organic Cathode and Its Electrochemical Performance
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
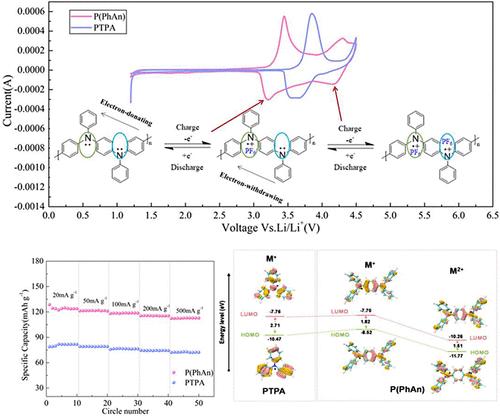
The growing demands for electronic devices and electric vehicles require the exploration of more efficient and high-power battery systems, in which the organic cathode materials are becoming a research hot issue in energy storage batteries. In the work, a polytriphenylamine derivative of polyphenylaniline (P(PhAn)) with a polyaniline-like molecular backbone structure was prepared by Buchwald-Hartwig C–N coupling reaction, and its electrochemical performances were evaluated in detail as the lithium organic cathode material. P(PhAn) as cathode displayed the multielectron redox characteristic, in which two pairs of redox characteristic peaks occurred in the measured voltage range. Density functional theory (DFT) calculation and simulations further proved that the formed push–pull electron effect and the conjugated polyaniline-like skeleton change the redox potentials and electron transfer of the cathode material. The electrode material also exhibited stable cycling performances and superior rate performances. It has a decent initial discharge specific capacity of 133 mAh·g–1, and after the 100 cycles, it kept at 111.2 mAh·g–1, remaining the 86% of capacity retention compared to the second cycle. Under the current densities of 20, 50, 100, 200, and 500 mA·g–1, it displayed the discharge specific capacities of 124, 122.1, 119.5, 115.3, and 112.4 mAh·g–1, respectively, and a 90.6% of capacity was even remained at the rate of 500 mA·g–1. Furthermore, it was proved that the energy storage process for P(PhAn) was mainly controlled by a capacitive-diffusion hybrid kinetics process.
多电子基聚苯基苯胺有机阴极的分子设计策略及其电化学性能
电子设备和电动汽车的需求日益增长,需要探索更高效、更高功率的电池系统,其中有机正极材料正成为储能电池的研究热点。该研究通过布赫瓦尔德-哈特维格 C-N 偶联反应制备了具有类聚苯胺分子骨架结构的聚三苯胺衍生物聚苯胺(P(PhAn)),并详细评估了其作为锂有机正极材料的电化学性能。作为阴极的 P(PhAn) 显示出多电子氧化还原特性,在测量电压范围内出现了两对氧化还原特性峰。密度泛函理论(DFT)计算和模拟进一步证明,形成的推拉电子效应和共轭聚苯胺样骨架改变了阴极材料的氧化还原电位和电子转移。该电极材料还表现出稳定的循环性能和优异的速率性能。它的初始放电比容量为 133 mAh-g-1,经过 100 次循环后,比容量保持在 111.2 mAh-g-1,与第二次循环相比,容量保持率为 86%。在 20、50、100、200 和 500 mA-g-1 的电流密度下,其放电比容量分别为 124、122.1、119.5、115.3 和 112.4 mAh-g-1,在 500 mA-g-1 的速率下,容量保持率甚至达到了 90.6%。此外,研究还证明,P(PhAn) 的储能过程主要由电容-扩散混合动力学过程控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


