Fe-Induced Surface Regulation and Accelerated Hydrogen Evolution Kinetics in γ-MnS Three-Dimensional Microarchitectures
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Exploration of earth-abundant, efficient, nonprecious-metal electrocatalysts with promising hydrogen evolution kinetics is crucial for electrochemical water-splitting technology. In this study, we present a promising iron-doped manganese sulfide electrocatalyst consisting of three-dimensional microarchitecture surfaces that exhibit efficient hydrogen evolution activity in an alkaline electrolyte. Iron doping induces surface regulation in MnS promoting the growth of various morphologies from 3D microarchitectures to 2D sheets. The 3D architecture, coupled with abundant active sites and iron incorporation, promotes hydrogen adsorption in manganese sulfide. The influence of iron doping on the hydrogen evolution activity of manganese sulfide was systematically investigated. The Mn0.95Fe0.05S electrocatalyst, with optimized iron incorporation, demonstrated a low overpotential of 147 mV to achieve a current density of 10 mA cm–2 in a 1 M KOH electrolyte. Post-hydrogen evolution reaction characterizations revealed Mn0.95Fe0.05S @FeMnOOHxSy, which were observed to be active catalysts enhancing the hydrogen evolution activity. This study presents an efficient strategy for Fe-induced 3D to 2D surface morphology modulation in MnS and offers an in-depth examination of its efficient electrochemical hydrogen evolution activity.
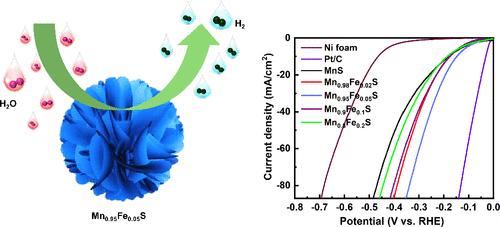
γ-MnS三维微结构中铁诱导的表面调节和加速氢演化动力学
探索具有良好氢进化动力学的富土、高效、非贵金属电催化剂对于电化学分水技术至关重要。在本研究中,我们提出了一种很有前景的铁掺杂硫化锰电催化剂,它由三维微结构表面组成,在碱性电解质中表现出高效的氢进化活性。铁掺杂诱导了硫化锰的表面调节,促进了从三维微架构到二维薄片的各种形态的生长。三维结构加上丰富的活性位点和铁的掺入,促进了硫化锰对氢的吸附。我们系统地研究了铁掺杂对硫化锰氢演化活性的影响。经过优化掺铁的 Mn0.95Fe0.05S 电催化剂在 1 M KOH 电解液中的过电位低至 147 mV,电流密度为 10 mA cm-2。氢进化反应后的表征显示,Mn0.95Fe0.05S @FeMnOOHxSy 是一种活性催化剂,可提高氢进化活性。本研究提出了一种铁诱导 MnS 从三维到二维表面形态调控的有效策略,并对其高效的电化学氢进化活性进行了深入研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


