In Vitro Screening for ToxCast Chemicals Binding to Thyroxine-Binding Globulin
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Thyroid hormone (TH) carrier proteins play an important role in distributing TH to target tissue as well as maintaining the balance of free versus bound TH in the blood. Interference with the TH carrier proteins has been identified as a potential mechanism of thyroid system disruption. To address the lack of data regarding chemicals binding to these carrier proteins and displacing TH, a fluorescence-based in vitro screening assay was utilized to screen over 1,400 chemicals from the U.S. EPA’s ToxCast phase1_v2, phase 2, and e1k libraries for competitive binding to one of the carrier proteins, thyroxine-binding globulin. Initial screening at a single high concentration of 100 μM identified 714 chemicals that decreased signal of the bound fluorescent ligand by 20% or higher. Of these, 297 produced 50% or greater reduction in fluorescence and were further tested in concentration–response (0.004 to 150 μM) to determine relative potency. Ten chemicals were found to have EC50 values <1 μM, 63 < 10 μM, and 141 chemicals between 10 and 100 μM. Utilization of this assay contributes to expanding the number of in vitro assays available for identifying chemicals with the potential to disrupt TH homeostasis. These results support ranking and prioritization of chemicals to be tested in vivo to aid in the development of a framework for predicting in vivo effects from in vitro high-throughput data.
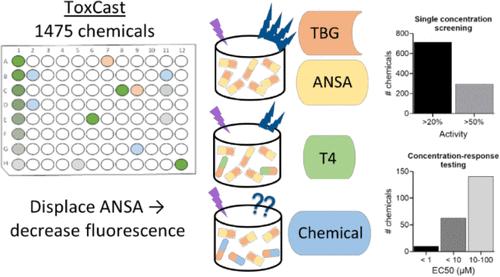
体外筛选与甲状腺素结合球蛋白结合的 ToxCast 化学品
甲状腺激素(TH)载体蛋白在将甲状腺激素分配到目标组织以及维持血液中游离与结合甲状腺激素的平衡方面发挥着重要作用。干扰甲状腺激素载体蛋白已被确定为甲状腺系统紊乱的潜在机制。为了解决有关化学物质与这些载体蛋白结合并置换 TH 的数据缺乏的问题,我们采用了一种基于荧光的体外筛选试验,从美国环保署的 ToxCast phase1_v2、phase 2 和 e1k 库中筛选出 1400 多种化学物质,以检测它们与载体蛋白之一甲状腺素结合球蛋白的竞争性结合。在 100 μM 的单一高浓度下进行的初步筛选发现,有 714 种化学物质会使结合荧光配体的信号降低 20% 或更多。其中,297 种化学物质可使荧光减少 50% 或更多,并进一步进行了浓度反应测试(0.004 至 150 μM),以确定其相对效力。结果发现,10 种化学物质的 EC50 值为 1 μM,63 种为 10 μM,141 种为 10 至 100 μM。利用这种检测方法有助于增加体外检测方法的数量,以确定有可能破坏总氢稳态的化学品。这些结果支持对需要进行体内测试的化学品进行排序和优先排序,以帮助开发一个框架,从体外高通量数据中预测体内效应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.30%
发文量
215
审稿时长
3.5 months
期刊介绍:
Chemical Research in Toxicology publishes Articles, Rapid Reports, Chemical Profiles, Reviews, Perspectives, Letters to the Editor, and ToxWatch on a wide range of topics in Toxicology that inform a chemical and molecular understanding and capacity to predict biological outcomes on the basis of structures and processes. The overarching goal of activities reported in the Journal are to provide knowledge and innovative approaches needed to promote intelligent solutions for human safety and ecosystem preservation. The journal emphasizes insight concerning mechanisms of toxicity over phenomenological observations. It upholds rigorous chemical, physical and mathematical standards for characterization and application of modern techniques.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


