Ag co-catalyst prepared by ultrasonic reduction method for efficient photocatalytic conversion of CO2 with H2O using ZnTa2O6 photocatalyst†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
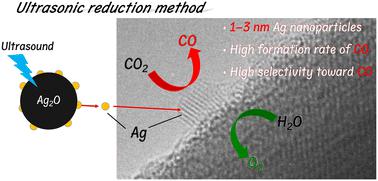
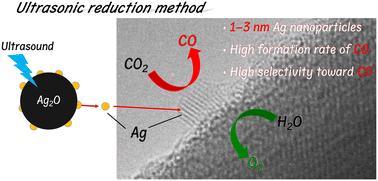
Towards the realisation of carbon neutrality by utilising renewable energy sources, the photocatalytic conversion of CO2 with H2O—known as artificial photosynthesis—is important because H2O is non-toxic or non-hazardous, and an abundant source of protons for CO2 reduction. Many studies on the photocatalytic conversion of CO2 have revealed that Ag nanoparticles are an effective co-catalyst for the selective conversion of CO2 to CO in water. To improve the activity of the photocatalytic conversion of CO2 in water, modifying the surface of the photocatalyst is essential to load small Ag nanoparticles with high dispersity, which is difficult to achieve using conventional methods. In this study, ultrasonic reduction (USR) was used as an advanced modification method for photocatalysts with an Ag co-catalyst. Ag/ZnTa2O6 prepared using the USR method exhibited good selectivity towards CO (>90%) evolution and a higher CO formation rate compared to those prepared using the conventional modification methods. High-resolution transmission electron microscopy images of the Ag co-catalyst revealed that Ag nanoparticles with a size of a single nanometre were loaded onto the surface of ZnTa2O6 by the USR method, whereas much larger Ag particles loaded onto it were observed in the case of other methods. Accordingly, a small Ag co-catalyst with a single nanometre size exhibits superior activity towards the selective conversion of CO2 to CO. Thus, we successfully achieved a high CO formation rate with high selectivity using a Ag/ZnTa2O6 photocatalyst prepared via USR.
利用 ZnTa2O6 光催化剂,通过超声还原法制备银助催化剂,实现 CO2 与 H2O 的高效光催化转化
为了利用可再生能源实现碳中和,二氧化碳与 H2O 的光催化转化(即人工光合作用)非常重要,因为 H2O 无毒无害,而且是用于还原二氧化碳的丰富质子源。许多关于 CO2 光催化转化的研究表明,Ag 纳米粒子是一种有效的辅助催化剂,可将水中的 CO2 有选择性地转化为 CO。为了提高光催化转化水中 CO2 的活性,必须对光催化剂表面进行改性,以负载高分散性的小尺寸银纳米粒子,而传统方法很难实现这一点。在本研究中,超声波还原(USR)作为一种先进的改性方法被用于带有银助催化剂的光催化剂。与使用传统改性方法制备的Ag/ZnTa2O6相比,使用USR方法制备的Ag/ZnTa2O6对CO(90%)演化具有良好的选择性,且CO形成率更高。银助催化剂的高分辨率透射电子显微镜图像显示,USR 法在 ZnTa2O6 表面载入的银纳米颗粒大小仅为一个纳米,而在其他方法中则观察到更大的银颗粒载入其中。因此,单纳米尺寸的小型银助催化剂在将 CO2 选择性转化为 CO 方面表现出更高的活性。因此,我们利用 USR 法制备的 Ag/ZnTa2O6 光催化剂成功地实现了高选择性和高 CO 生成率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


