1H, 13C, 15N NMR, and DFT Studies on Complex Formation of Zinc(II) Ion with Ethylenediamine in Ionic Liquid [C2mIm][TFSA]
IF 2.8
2区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
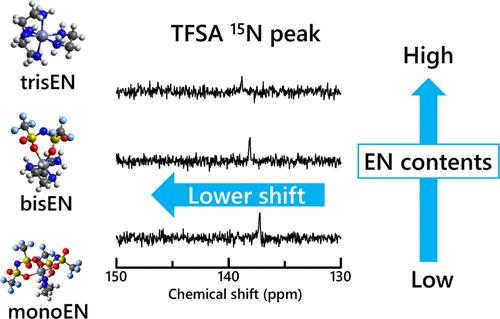
In bis(trifluoromethylsulfonyl)amide (TFSA–)-based ionic liquid (IL), 1-ethyl-3-methylimidazolium TFSA– ([C2mIm][TFSA]), the complex formation equilibria of zinc(II) ion (Zn2+) with ethylenediamine (EN) have been investigated. An EN molecule may coordinate with Zn2+ as a bidentate ligand. First, the formation of Zn2+–EN complexes in [C2mIm][TFSA] was confirmed from the difference of 1H and 13C NMR chemical shift values of EN molecules between [C2mIm][TFSA]–EN binary solvents and the 0.1 mol dm–3 Zn(TFSA)2/[C2mIm][TFSA]–EN solutions as a function of EN mole fraction xEN. Second, the stability constants of Zn2+–EN complexes formed in the IL were determined from the concentration ratio [EN]/[Zn2+] dependence of 15N NMR chemical shift values of the TFSA– N atom in the Zn2+/IL–EN solutions. In the IL, mono-, bis-, and tris-EN complexes are successively formed by 1:1 replacement of TFSA– anions coordinated with Zn2+ by EN molecules with increasing EN content. Third, 1H and 13C NMR measurements with the help of density functional theory (DFT) calculations were made on [C2mIm][TFSA]–EN binary solvents as a function of xEN to clarify key interactions to the mechanism of the complex formation. Fourth, the stability constants of Zn2+–EN complexes in the IL were compared with those in aqueous solutions. It was suggested that the hydrogen bonding of the EN molecule with the imidazolium ring H atoms and the TFSA– O atoms reduces the stability of the mono-EN complex in the IL. In contrast, the intracomplex hydrogen bonds between EN and TFSA– in the first coordination shell contribute to the higher stability of the bis-EN complex in the IL than that in aqueous solutions. The difference in the stability constants between the tris-EN complexes and hexaacetonitrile complexes, where acetonitrile (AN) molecules act as monodentate ligands, was interpreted in terms of the higher electron donicity of EN. Finally, to verify the present evaluation, the experimental 13C NMR chemical shift values of EN molecules in the solutions were compared with the theoretical values calculated by DFT using the stability constants determined.
离子液体 [C2mIm][TFSA]中锌(II) 离子与乙二胺形成络合物的 1H、13C、15N NMR 和 DFT 研究
在双(三氟甲基磺酰基)酰胺(TFSA-)基离子液体(IL)1-乙基-3-甲基咪唑鎓 TFSA-([C2mIm][TFSA])中,研究了锌(II)离子(Zn2+)与乙二胺(EN)的络合物形成平衡。乙二胺分子可作为双齿配体与 Zn2+ 配位。首先,[C2mIm][TFSA]-EN 二元溶剂和 0.1 mol dm-3 Zn(TFSA)2/[C2mIm][TFSA]-EN 溶液中 EN 分子的 1H 和 13C NMR 化学位移值的差异与 EN 分子分数 xEN 的函数关系证实了 Zn2+-EN 复合物在[C2mIm][TFSA]中的形成。其次,根据 Zn2+/IL-EN 溶液中 TFSA- N 原子的 15N NMR 化学位移值与浓度比 [EN]/[Zn2+] 的关系,确定了在 IL 中形成的 Zn2+-EN 复合物的稳定常数。在 IL 中,随着 EN 含量的增加,与 Zn2+ 配位的 TFSA- 阴离子会被 EN 分子以 1:1 的比例取代,从而相继形成单-EN、双-EN 和三-EN 复合物。第三,在密度泛函理论(DFT)计算的帮助下,对[C2mIm][TFSA]-EN 双元溶剂中的 xEN 进行了 1H 和 13C NMR 测量,以阐明复合物形成机制的关键相互作用。第四,比较了 Zn2+-EN 复合物在 IL 与水溶液中的稳定性常数。研究表明,EN 分子与咪唑环 H 原子和 TFSA- O 原子的氢键作用降低了单-EN 复合物在 IL 中的稳定性。相反,EN 与第一配位层中的 TFSA- 之间的复合物内氢键使双-EN 复合物在 IL 中的稳定性高于在水溶液中的稳定性。三-EN 复合物与六乙腈复合物(其中乙腈(AN)分子充当单齿配体)之间在稳定性常数上的差异被解释为 EN 具有更高的电子捐献性。最后,为了验证本评估结果,将溶液中 EN 分子的 13C NMR 化学位移实验值与 DFT 利用所确定的稳定常数计算出的理论值进行了比较。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.80
自引率
9.10%
发文量
965
审稿时长
1.6 months
期刊介绍:
An essential criterion for acceptance of research articles in the journal is that they provide new physical insight. Please refer to the New Physical Insights virtual issue on what constitutes new physical insight. Manuscripts that are essentially reporting data or applications of data are, in general, not suitable for publication in JPC B.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


