Self-Assembled Iron(III) Coordination Cage as an MRI-Active Carrier for a Gold(I) Drug
引用次数: 0
Abstract
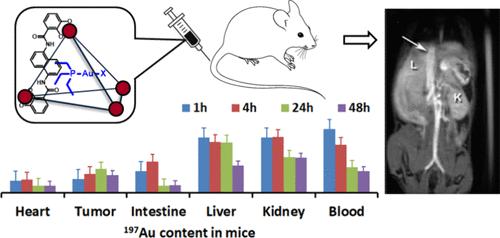
A T1 MRI probe based on a self-assembled coordination cage with four iron(III) centers acts as a host for the hydrolysis product of the gold(I) anticancer drug, Au(PEt3)Cl. 1H NMR characterization of the gold complex encapsulated within the diamagnetic Ga(III) analog of the coordination cage is consistent with loss of chloride to give aquated gold complex, most likely [Au(PEt3)(OH2)]+ within the cage. The gold complex undergoes pH-dependent speciation changes in the Ga(III) cage and is released at mildly acidic pH from both the Ga(III) and Fe(III) cages. NMR spectroscopy studies of the encapsulated gold complex in the presence of human serum albumin (HSA) show that the gold complex remains inside of the Ga(III) cage for several hours, resisting release and binding to cysteine residues of HSA. The Fe(III) cage with encapsulated gold complex shows enhanced contrast of the vasculature and uptake into CT26 tumors in BALB/c mice as shown by MRI. The gold complex is solubilized by the iron(III) cage for intravenous injection, whereas the free complex must be injected intraperitoneally. Gold complex accumulates in the tumor for both caged and free complex over 1–48 h as measured by ex-vivo analysis. Encapsulation in the Fe(III) cage modulates the biodistribution of the gold complex in mice in comparison to the free complex, consistent with the function of the cage as a carrier.
作为金(I)药物磁共振成像活性载体的自组装铁(III)配位笼
T1 核磁共振成像探针基于一个具有四个铁(III)中心的自组装配位笼,可作为金(I)抗癌药物 Au(PEt3)Cl 的水解产物的宿主。对包裹在配位笼的二磁性 Ga(III)类似物中的金复合物进行的 1H NMR 表征表明,氯化物流失后,在配位笼中产生了含水金复合物,很可能是[Au(PEt3)(OH2)]+。金配合物在 Ga(III)笼中发生了随 pH 值变化的标示变化,并在 pH 值呈弱酸性时从 Ga(III)笼和 Fe(III)笼中释放出来。在人血清白蛋白(HSA)存在下对封装的金复合物进行的核磁共振光谱研究表明,金复合物在 Ga(III) 笼内保持了几个小时,不易释放并与 HSA 的半胱氨酸残基结合。核磁共振成像显示,封装了金复合物的铁(III)笼增强了BALB/c小鼠血管的对比度和对CT26肿瘤的吸收。金复合物被铁(III)笼溶解后可进行静脉注射,而游离复合物则必须进行腹腔注射。根据体外分析,笼状和游离复合物的金复合物都会在肿瘤中积累 1-48 小时。与游离复合物相比,封装在铁(III)笼中会改变金复合物在小鼠体内的生物分布,这与铁(III)笼作为载体的功能是一致的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


