Prevalence, trends, and distribution of hepatitis C virus among the general population in sub-Saharan Africa: A systematic review and meta-analysis
Abstract
Background and Aims
Although the evidence is uncertain, existing estimates for hepatitis C virus (HCV) in sub-Saharan Africa (SSA) indicate a high burden. We estimated HCV seroprevalence and viraemic prevalence among the general population in SSA.
Methods
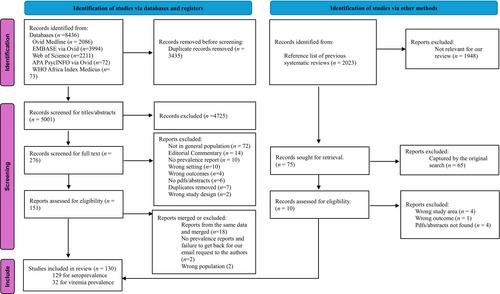
We searched Medline, Embase, Web of Science, APA PsycINFO, and World Health Organization Africa Index Medicus for community-based studies. Study quality was assessed using the Joanna Briggs Institute critical appraisal tool, and heterogeneity using the index of heterogeneity (I2). Two approaches were deployed. First, we used random-effects meta-analysis to pool prevalence. Second, to derive representative estimates, we weighted each country's HCV seroprevalence using 2021 United Nations country population sizes.
Results
We synthesized 130 studies. Overall, SSA HCV seroprevalence from the random-effects model was 4.17% (95% confidence interval [CI]: 3.71–4.66, I2 = 99.30%). There were no differences between males (4.31%) and females (4.03%). Seroprevalence was 2.25%, 3.31%, and 16.23% for ages ≤20, 21–64, and ≥65 years, respectively, and was higher in rural (6.63%) versus urban (2.93%). There was indication of decrement overtime from 5.74% to 4.35% to 3.03% in the years 1984–2000, 2001–2014, and 2015–2023, respectively. The weighted overall SSA HCV seroprevalence was estimated to be 2.30% (95% CI: 1.59–3.00) with regional variation: Africa-Southern (.79%), Africa-Central (1.47%), Africa-Eastern (2.71%), and Africa-Western (2.88%). HCV viremia among HCV seropositives was 54.77% (95% CI: 47.80–61.66).
Conclusions
HCV seroprevalence in SSA remains high. Populations aged ≥65 years, rural communities, Africa-Western, and some countries in Africa-Central and Africa-Eastern appear disproportionately affected. These results underline the need for governmental commitment to achieve the 2030 global HCV elimination targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


