High Selective Electrocatalysis Dehydrogenation of Isopropanol to Acetone with Cobenefits: Carboxylic Acids Coproduction
引用次数: 0
Abstract
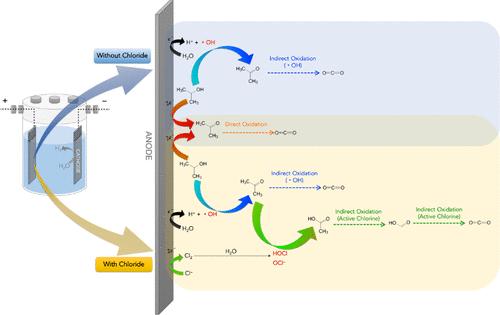
The existing on-site treatment of residual 2-propanol (IPA) in semiconductor factories results in evaporation into the atmosphere, causing cross-contamination of water and air pollution. Various treatment technologies have been assessed, but many either generate pollution or cannot recover IPA. Alternatively, IPA undergoes oxidation during distillation, transforming into acetone, another substance regulated under wastewater treatment standards. This study explored electrochemical oxidation (EO) as a method for selectively mineralizing IPA in wastewater. The high flow rate and complex byproducts of IPA wastewater necessitate advanced approaches for efficient treatment. Employing a well-enclosed EO reactor, this research characterized radical and active chlorine species in depth, elucidating their composition, mechanisms, and roles in removing IPA and its intermediates. Hydroxyl radicals (•OH) were identified as the most reactive species, as they fully removed IPA in 5 h in a chloride-free system. The introduction of electrogenerated active chlorine species proved to be highly efficient for treatment, especially in a 150 mM NaCl electrolyte at an initial pH of 5, which is suitable for wastewater containing high chlorine concentrations. This approach not only effectively mitigates acetone generation but also enhances IPA mineralization, presenting a viable treatment option without the need for additional chemicals.
高选择性电催化异丙醇脱氢为丙酮,同时具有增效作用:羧酸共生
半导体工厂现有的现场处理残留 2-丙醇(IPA)的方法会导致蒸发到大气中,造成水和空气的交叉污染。已对各种处理技术进行了评估,但许多技术要么会产生污染,要么无法回收 IPA。另外,IPA 在蒸馏过程中会发生氧化,转化为丙酮,而丙酮是另一种受废水处理标准管制的物质。本研究探讨了电化学氧化(EO)作为一种选择性矿化废水中异丙醇的方法。由于异丙醇废水流量大、副产物复杂,因此有必要采用先进的方法进行高效处理。本研究采用封闭式环氧乙烷反应器,对自由基和活性氯物种进行了深入研究,阐明了它们的组成、机理以及在去除异丙醇及其中间产物中的作用。羟基自由基(-OH)被认为是反应最活跃的物种,因为它们在无氯系统中 5 小时内就能完全去除异丙醇。事实证明,引入电生成的活性氯物种具有很高的处理效率,尤其是在初始 pH 值为 5 的 150 mM NaCl 电解质中,这种处理方法适用于含氯浓度较高的废水。这种方法不仅能有效减少丙酮的生成,还能增强 IPA 矿化,是一种无需额外化学品的可行处理方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


