The tumor suppressor Parkin exerts anticancer effects through regulating mitochondrial GAPDH activity
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cancer cells preferentially utilize glycolysis for energy production, and GAPDH is a critical enzyme in glycolysis. Parkin is a tumor suppressor and a key protein involved in mitophagy regulation. However, the tumor suppression mechanism of Parkin has still not been elucidated. In this study, we identified mitochondrial GAPDH as a new substrate of the E3 ubiquitin ligase Parkin, which mediated GAPDH ubiquitination in human cervical cancer. The translocation of GAPDH into mitochondria was driven by the PINK1 kinase, and either PINK1 or GAPDH mutation prevented the accumulation of GAPDH in mitochondria. Parkin caused the ubiquitination of GAPDH at multiple sites (K186, K215, and K219) located within the enzyme-catalyzed binding domain of the GAPDH protein. GAPDH ubiquitination was required for mitophagy, and stimulation of mitophagy suppressed cervical cancer cell growth, indicating that mitophagy serves as a type of cell death. Mechanistically, PHB2 served as a key mediator in GAPDH ubiquitination-induced mitophagy through stabilizing PINK1 protein and GAPDH mutation resulted in the reduced distribution of PHB2 in mitophagic vacuole. In addition, ubiquitination of GAPDH decreased its phosphorylation level and enzyme activity and inhibited the glycolytic pathway in cervical cancer cells. The results of in vivo experiments also showed that the GAPDH mutation increased glycolysis in cervical cancer cells and accelerated tumorigenesis. Thus, we concluded that Parkin may exert its anticancer function by ubiquitinating GAPDH in mitochondria. Taken together, our study further clarified the molecular mechanism of tumor suppression by Parkin through the regulation of energy metabolism, which provides an experimental basis for the development of new drugs for the treatment of human cervical cancer.

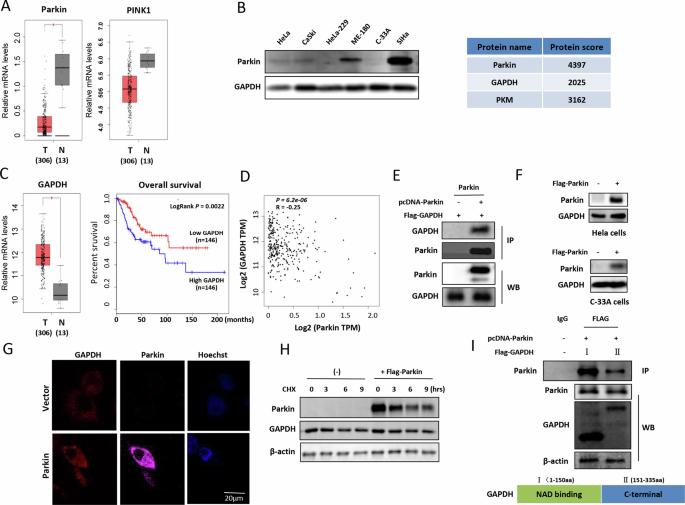
肿瘤抑制因子帕金通过调节线粒体 GAPDH 活性发挥抗癌作用
癌细胞优先利用糖酵解产生能量,而 GAPDH 是糖酵解过程中的一种关键酶。Parkin 是一种肿瘤抑制因子,也是参与有丝分裂调节的关键蛋白。然而,Parkin 的抑瘤机制尚未阐明。在这项研究中,我们发现线粒体 GAPDH 是 E3 泛素连接酶 Parkin 的新底物,Parkin 在人类宫颈癌中介导了 GAPDH 泛素化。GAPDH转位到线粒体是由PINK1激酶驱动的,PINK1或GAPDH突变都会阻止GAPDH在线粒体中的积累。Parkin 在 GAPDH 蛋白的酶催化结合域内的多个位点(K186、K215 和 K219)上导致 GAPDH 泛素化。有丝分裂需要 GAPDH 泛素化,刺激有丝分裂可抑制宫颈癌细胞的生长,这表明有丝分裂是细胞死亡的一种类型。从机理上讲,PHB2通过稳定PINK1蛋白而成为GAPDH泛素化诱导有丝分裂的关键介质,GAPDH突变导致PHB2在有丝分裂液泡中的分布减少。此外,GAPDH 泛素化降低了其磷酸化水平和酶活性,抑制了宫颈癌细胞的糖酵解途径。体内实验结果也表明,GAPDH 突变会增加宫颈癌细胞中的糖酵解,加速肿瘤发生。因此,我们得出结论,Parkin 可能通过泛素化线粒体中的 GAPDH 来发挥其抗癌功能。综上所述,我们的研究进一步阐明了Parkin通过调控能量代谢抑制肿瘤的分子机制,为开发治疗人类宫颈癌的新药提供了实验依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


