SUV39H1 epigenetically modulates the MCPIP1-AURKA signaling axis to enhance neuroblastoma tumorigenesis
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
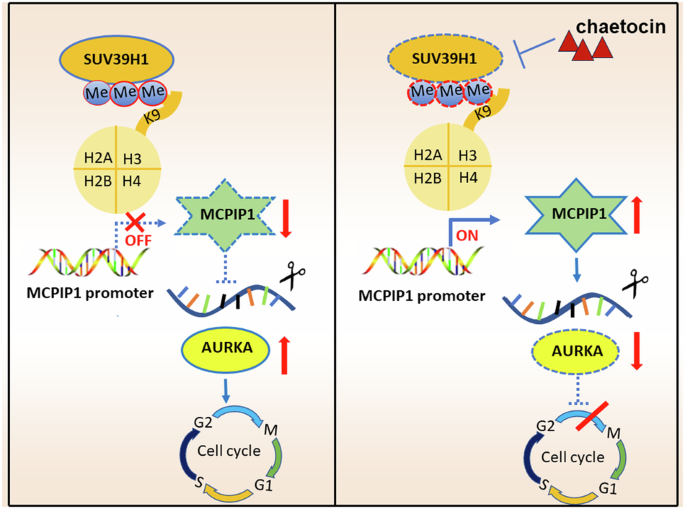
Epigenetic regulation is a pivotal factor during neuroblastoma (NB) pathogenesis and investigations into cancer epigenetics are actively underway to identify novel therapeutic strategies for NB patients. SUV39H1, a member of the H3K9 methyltransferase family, contributing to tumorigenesis across multiple malignancies. However, its specific role in NB remains unexplored. In this study, we conducted a high-throughput screen utilizing a compound library containing 288 epigenetic drugs, leading to the identification of chaetocin as the most potent NB inhibitor by targeting SUV39H1. Genetic manipulation and therapeutic inhibition of SUV39H1 significantly impacted proliferation, migration, cell cycle phases, and apoptosis in NB cells. Concurrently, chaetocin demonstrated robust anti-tumor efficacy in vivo with tolerable toxicity. RNA-seq unveiled that SUV39H1 knockdown and inhibition down-regulated cell cycle pathways, impacting vital genes such as AURKA. Besides, MCPIP1 emerged as a novel tumor suppressor following SUV39H1 inhibition, which decreased AURKA expression in NB. In detail, SUV39H1 mediated the enrichment of H3K9me3 at the promoter region of MCPIP1, repressing the MCPIP1-mediated degradation of AURKA and facilitating the subsequent accumulation of AURKA, which revealed the oncogenic role of SUV39H1 via the SUV39H1-MCPIP1-AURKA signaling axis in NB. Therapeutic inhibition of SUV39H1 using chaetocin emerges as an effective and safe strategy for NB patients.
SUV39H1 表观遗传修饰 MCPIP1-AURKA 信号轴,促进神经母细胞瘤的肿瘤发生
表观遗传调控是神经母细胞瘤(NB)发病过程中的一个关键因素,目前正在积极研究癌症表观遗传学,以便为 NB 患者找出新的治疗策略。SUV39H1 是 H3K9 甲基转移酶家族的成员,在多种恶性肿瘤的肿瘤发生过程中起着重要作用。然而,它在 NB 中的具体作用仍未得到探索。在这项研究中,我们利用一个包含288种表观遗传药物的化合物库进行了高通量筛选,最终通过靶向SUV39H1鉴定出柴胡素是最有效的NB抑制剂。对SUV39H1的遗传操作和治疗抑制显著影响了NB细胞的增殖、迁移、细胞周期阶段和凋亡。同时,chaetocin在体内显示出强大的抗肿瘤功效,且毒性可耐受。RNA-seq发现,敲除和抑制SUV39H1会下调细胞周期通路,影响AURKA等重要基因。此外,在抑制 SUV39H1 后,MCPIP1 成为一种新的肿瘤抑制因子,它降低了 AURKA 在 NB 中的表达。具体而言,SUV39H1介导了MCPIP1启动子区H3K9me3的富集,抑制了MCPIP1介导的AURKA降解,促进了AURKA的后续积累,从而揭示了SUV39H1通过SUV39H1-MCPIP1-AURKA信号轴在NB中的致癌作用。使用柴胡素治疗性抑制SUV39H1是治疗NB患者的一种有效而安全的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


