Single-cell RNA-sequencing reveals a unique landscape of the tumor microenvironment in obesity-associated breast cancer
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
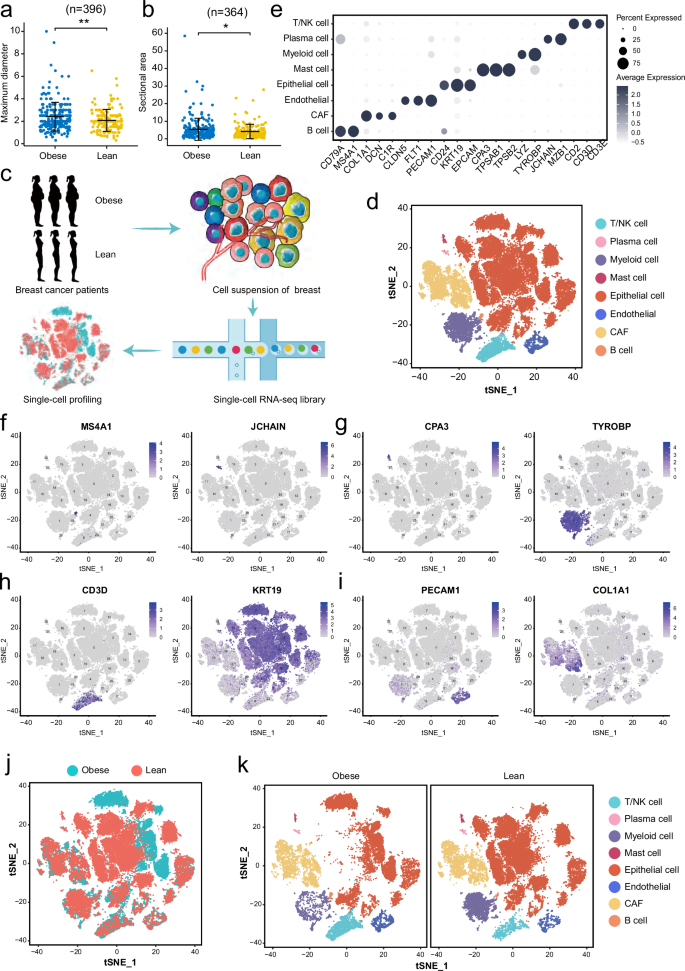
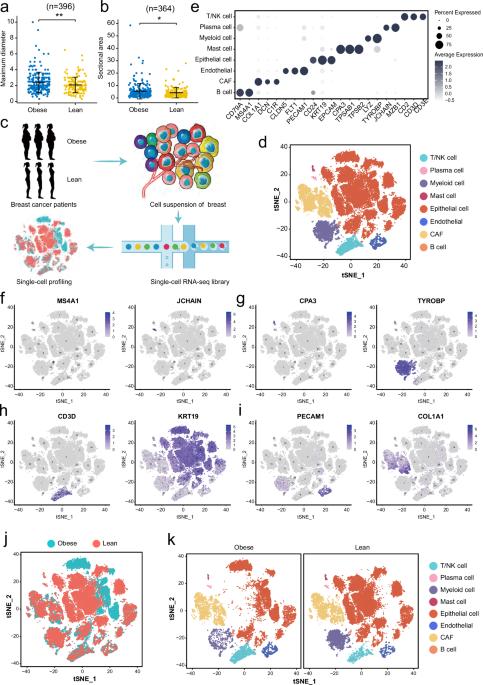
As two diseases with rapidly increasing incidence, the molecular linkages between obesity and breast cancer (BC) are intriguing. Overall, obesity may be a negative prognostic factor for BC. Single-cell RNA-sequencing (scRNA-seq) was performed on tumor tissues from 6 obese and non-obese BC patients. With 48,033 cells analyzed, we found heterogeneous tumor epithelium and microenvironment in these obese and lean BC patients. Interestingly, the obesity-associated epithelial cells exhibited specific expression signatures which linked tumor growth and hormone metabolism in BC. Notably, one population of obesity-specific macrophage up-regulated the nuclear receptor subfamily 1 group H member 3 (NR1H3), which acted a transcription factor and regulated FABP4 expression through its interaction with the DNA of SREBP1, and further increased the proliferation of tumor cells in BC. Using single-cell signatures, our study illustrate cell diversity and transcriptomic differences in tumors from obese and non-obese BC patients, and sheds light on potential molecular link between lipid metabolism and BC.
单细胞 RNA 测序揭示肥胖相关乳腺癌肿瘤微环境的独特面貌
作为发病率迅速上升的两种疾病,肥胖与乳腺癌(BC)之间的分子联系令人好奇。总体而言,肥胖可能是乳腺癌的一个负面预后因素。研究人员对6名肥胖和非肥胖乳腺癌患者的肿瘤组织进行了单细胞RNA测序(scRNA-seq)。我们分析了48,033个细胞,发现这些肥胖和瘦弱的BC患者的肿瘤上皮和微环境存在异质性。有趣的是,肥胖相关的上皮细胞表现出特定的表达特征,这与肿瘤生长和 BC 激素代谢有关。值得注意的是,肥胖特异性巨噬细胞群体上调了核受体1亚家族H组3(NR1H3),NR1H3是一种转录因子,通过与SREBP1的DNA相互作用调节FABP4的表达,并进一步增加了BC肿瘤细胞的增殖。我们的研究利用单细胞特征说明了肥胖和非肥胖BC患者肿瘤细胞的多样性和转录组学差异,并揭示了脂质代谢与BC之间的潜在分子联系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


