A Small Molecule Impedes the Aβ1–42 Tetramer Neurotoxicity by Preserving Membrane Integrity: Microsecond Multiscale Simulations
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Amyloid-β (Aβ1–42) peptides aggregated into plaques deposited in the brain are the main hallmark of Alzheimer’s disease (AD), a social and economic burden worldwide. In this context, insoluble Aβ1–42 fibrils are the main components of plaques. The recent trials that used approved AD drugs show that they can remove the fibrils from AD patients’ brains, but they did not halt the course of the disease. Mounting evidence envisages that the soluble Aβ1–42 oligomers’ interactions with the neuronal membrane trigger higher cell death than Aβ1–42 fibril interactions. Developing a compound that can alleviate the oligomer’s toxicity is one of the most demanding tasks for curing the disease. We performed two molecular dynamics (MD) simulations in an explicit solvent model. In the first case, 55-μs of multiscale all-atom (AA)/coarse-grained (CG) MD simulations were carried out to decipher the impact of a previously described small anti-Aβ molecule, termed M30 (2-octahydroisoquinolin-2(1H)-ylethanamine), on an Aβ1–42 tetramer structure in close contact with a DMPC bilayer. In the second case, 15-μs AA/CG MD simulations were performed to rationalize the dynamics between Aβ1–42 and Aβ1–42-M30 tetramer complexes embedded in DMPC. On the membrane bilayer, we found that the Aβ1–42 tetramer penetrates the bilayer surface due to unrestricted conformational flexibility and many contacts with the membrane phosphate groups. In contrast, no Aβ1–42-M30 tetramer penetration was observed during the entire course of the simulation. In the case of the membrane-embedded Aβ1–42 tetramer, the integrity of the bottom bilayer leaflet was severely affected by the interactions between the negatively charged phosphate groups and the positively charged residues of the Aβ1–42 tetramer, resulting in a deep tetramer penetration into the bilayer hydrophobic region. These contacts were not observed in the case of the membrane-embedded Aβ1–42-M30 tetramer. It was noted that M30 molecules bind to Aβ1–42 tetramer through hydrogen bonds, resulting in a conformational stable Aβ1–42-M30 complex. The associated complex has reduced conformational changes and an enhanced rigidity that prevents the tetramer dissociation by interfering with the tetramer-membrane contacts. Our findings suggest that the M30 molecules could bind to Aβ1–42 tetramer resulting in a rigid structure, and that such complexes do not significantly perturb the membrane bilayer organization. These observations support the in vitro and in vivo experimental evidence that the M30 molecules prevent synaptotocity, improving AD-affected mice memory.
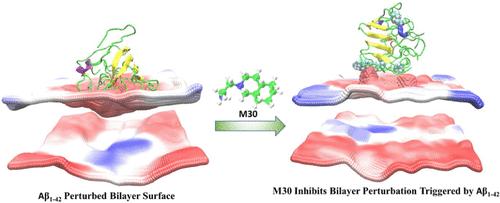
一种小分子通过保持膜完整性来抑制 Aβ1-42 四聚体的神经毒性:微秒级多尺度模拟
淀粉样蛋白-β(Aβ1-42)肽聚集成斑块沉积在大脑中,是阿尔茨海默病(AD)的主要特征,也是全世界的社会和经济负担。在这种情况下,不溶性 Aβ1-42 纤维是斑块的主要成分。最近使用已获批准的抗阿尔茨海默病药物进行的试验表明,这些药物可以清除阿尔茨海默病患者大脑中的纤维,但并不能阻止疾病的发展。越来越多的证据表明,与Aβ1-42纤维相互作用相比,可溶性Aβ1-42低聚物与神经元膜的相互作用会引发更多的细胞死亡。开发一种能减轻寡聚体毒性的化合物是治疗这种疾病最艰巨的任务之一。我们在显式溶剂模型中进行了两次分子动力学(MD)模拟。在第一种情况下,我们进行了 55μs 的多尺度全原子(AA)/粗粒度(CG)MD 模拟,以破解之前描述过的抗 Aβ 小分子(称为 M30(2-八氢异喹啉-2(1H)-乙胺))对与 DMPC 双层紧密接触的 Aβ1-42 四聚体结构的影响。在第二种情况下,我们进行了 15-μs AA/CG MD 模拟,以合理解释嵌入 DMPC 的 Aβ1-42 和 Aβ1-42-M30 四聚体复合物之间的动力学关系。 在膜双分子层上,我们发现 Aβ1-42 四聚体由于不受限制的构象灵活性和与膜磷酸基团的多次接触而穿透了双分子层表面。相反,在整个模拟过程中没有观察到 Aβ1-42-M30 四聚体穿透。在膜嵌入 Aβ1-42 四聚体的情况下,由于带负电荷的磷酸基团和 Aβ1-42 四聚体带正电荷的残基之间的相互作用,双分子层底部小叶的完整性受到严重影响,导致四聚体深入双分子层疏水区域。在膜包埋的 Aβ1-42-M30 四聚体中则没有观察到这些接触。研究发现,M30 分子通过氢键与 Aβ1-42 四聚体结合,形成构象稳定的 Aβ1-42-M30 复合物。相关的复合物减少了构象变化,增强了刚性,通过干扰四聚体与膜的接触防止四聚体解离。我们的研究结果表明,M30 分子可以与 Aβ1-42 四聚体结合,从而形成刚性结构,而且这种复合物不会对膜双分子层的组织结构造成显著干扰。这些观察结果支持了体外和体内实验证据,即 M30 分子可防止突触加速,改善受 AD 影响的小鼠记忆。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


