Deep learning-based prediction of the dose–volume histograms for volumetric modulated arc therapy of left-sided breast cancer
Abstract
Background
The advancements in artificial intelligence and computational power have made deep learning an attractive tool for radiotherapy treatment planning. Deep learning has the potential to significantly simplify the trial-and-error process involved in inverse planning required by modern treatment techniques such as volumetric modulated arc therapy (VMAT). In this study, we explore the ability of deep learning to predict organ-at-risk (OAR) dose–volume histograms (DVHs) of left-sided breast cancer patients undergoing VMAT treatment based solely on their anatomical characteristics. The predicted DVHs could be used to derive patient-specific dose constraints and dose objectives, streamlining the treatment planning process, standardizing the quality of the plans, and personalizing the treatment planning.
Purpose
This study aimed to develop a deep learning-based framework for the prediction of organ-specific dose–volume histograms (DVH) based on structures delineated for left-sided breast cancer treatment.
Methods
We used a dataset of 249 left-sided breast cancer patients treated with tangential VMAT fields. We extracted delineated structures and dose distributions for each patient and derived slice-by-slice DVHs for planning target volume (PTV) and organs-at-risk. The patients were divided into training (70%, n = 174), validation (10%, n = 24), and test (20%, n = 51) sets. Collected data were used to train a deep learning model for the prediction of the DVHs based on the delineated structures. The developed deep learning model comprised a modified DenseNet architecture followed by a recurrent neural network.
Results
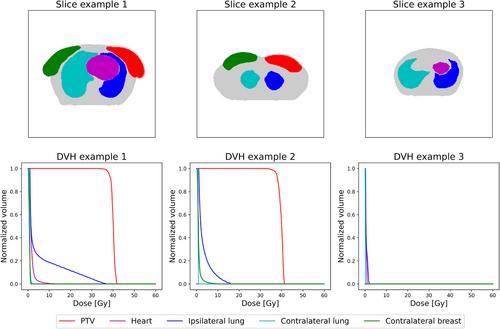
In the independent test set (n = 51), the point-wise differences in the slice-by-slice DVHs between the clinical and predicted DVHs were small; the mean squared errors were 3.53, 1.58, 2.28, 3.37, and 1.44 [×10−4] for PTV, heart, ipsilateral lung, contralateral lung, and contralateral breast, respectively. With the derived cumulative DVHs, the mean absolute difference ± standard deviation of mean doses between the clinical and the predicted DVH were 0.08 ± 0.04 Gy, 0.24 ± 0.22 Gy, 0.73 ± 0.46 Gy, 0.07 ± 0.06 Gy, and 0.14 ± 0.14 Gy for PTV, heart, ipsilateral lung, contralateral lung, and contralateral breast, respectively.
Conclusions
The deep learning-based approach enabled automatic and reliable prediction of the DVH based on delineated structures. The predicted DVHs could potentially serve as patient-specific clinical goals used to aid treatment planning and avoid suboptimal plans or to derive optimization objectives and constraints for automated treatment planning.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


