A microscopic oxygen transport model for ultra-high dose rate radiotherapy in vivo: The impact of physiological conditions on FLASH effect
Abstract
Background
Ultra-high dose rate irradiation (≥40 Gy/s, FLASH) has been shown to reduce normal tissue toxicity, while maintaining tumor control compared to conventional dose-rate radiotherapy. The radiolytic oxygen (O2) depletion (ROD) resulting from FLASH has been proposed to explain the normal tissue protection effect; however, in vivo experiments have not confirmed that FLASH induced global tissue hypoxia. Nonetheless, the experiments reported are based on volume-averaged measurement, which have inherent limitations in detecting microscopic phenomena, including the potential preservation of stem cells niches due to local FLASH-induced O2 depletion. Computational modeling offers a complementary approach to understand the ROD caused by FLASH at the microscopic level.
Purpose
We developed a comprehensive model to describe the spatial and temporal dynamics of O2 consumption and transport in response to irradiation in vivo. The change of oxygen enhancement ratio (OER) was used to quantify and investigate the FLASH effect as a function of physiological and radiation parameters at microscopic scale.
Methods
We considered time-dependent O2 supply and consumption in a 3D cylindrical geometry, incorporating blood flow linking the O2 concentration ([O2]) in the capillary to that within the tissue through the Hill equation, radial and axial diffusion of O2, metabolic and zero-order radiolytic O2 consumption, and a pulsed radiation structure. Time-evolved distributions of [O2] were obtained by numerically solving perfusion-diffusion equations. The model enables the computation of dynamic O2 distribution and the relative change of OER (δROD) under various physiological and radiation conditions in vivo.
Results
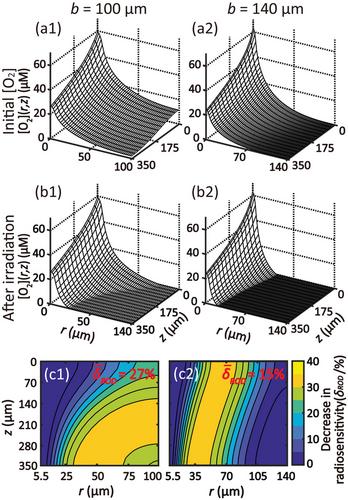
Initial [O2] level and the subsequent changes during irradiation determined δROD distribution, which strongly depends on physiological parameters, i.e., intercapillary spacing, ultimately determining the tissue area with enhanced radioresistance. We observed that the δROD/FLASH effect is affected by and sensitive to the interplay effect among physiological and radiation parameters. It renders that the FLASH effect can be tissue environment dependent. The saturation of FLASH normal tissue protection upon dose and dose rate was shown. Beyond ∼60 Gy/s, no significant decrease in radiosensitivity within tissue region was observed. In turn, for a given dose rate, the change of radiosensitivity became saturated after a certain dose level. Pulse structures with the same dose and instantaneous dose rate but with different delivery times were shown to have distinguishable δROD thus tissue sparing, suggesting the average dose rate could be a metric assessing the FLASH effect and demonstrating the capability of our model to support experimental findings.
Conclusion
On a macroscopic scale, the modeling results align with the experimental findings in terms of dose and dose rate thresholds, and it also indicates that pulse structure can vary the FLASH effect. At the microscopic level, this model enables us to examine the spatially resolved FLASH effect based on physiological and irradiation parameters. Our model thus provides a complementary approach to experimental methods for understanding the underlying mechanism of FLASH radiotherapy. Our results show that physiological conditions can potentially determine the FLASH efficacy in tissue protection. The FLASH effect may be observed under optimal combination of physiological parameters, not limited to radiation conditions alone.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


