Enteroids to Study Pediatric Intestinal Drug Transport
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Intestinal maturational changes after birth affect the pharmacokinetics (PK) of drugs, having major implications for drug safety and efficacy. However, little is known about ontogeny-related PK patterns in the intestine. To explore the accuracy of human enteroid monolayers for studying drug transport in the pediatric intestine, we compared the drug transporter functionality and expression in enteroid monolayers and tissue from pediatrics and adults. Enteroid monolayers were cultured of 14 pediatric [median (range) age: 44 weeks (2 days–13 years)] and 5 adult donors, in which bidirectional drug transport experiments were performed. In parallel, we performed similar experiments with tissue explants in Ussing chamber using 11 pediatric [median (range) age: 54 weeks (15 weeks–10 years)] and 6 adult tissues. Enalaprilat, propranolol, talinolol, and rosuvastatin were used to test paracellular, transcellular, and transporter-mediated efflux by P-gp and breast cancer resistance protein (BCRP), respectively. In addition, we compared the expression patterns of ADME-related genes in pediatric and adult enteroid monolayers with tissues using RNA sequencing. Efflux transport by P-gp and BCRP was comparable between the enteroids and tissue. Efflux ratios (ERs) of talinolol and rosuvastatin by P-gp and BCRP, respectively, were higher in enteroid monolayers compared to Ussing chamber, likely caused by experimental differences in model setup and cellular layers present. Explorative statistics on the correlation with age showed trends of increasing ER with age for P-gp in enteroid monolayers; however, it was not significant. In the Ussing chamber setup, lower enalaprilat and propranolol transport was observed with age. Importantly, the RNA sequencing pathway analysis revealed that age-related variation in drug metabolism between neonates and adults was present in both enteroids and intestinal tissue. Age-related differences between 0 and 6 months old and adults were observed in tissue as well as in enteroid monolayers, although to a lesser extent. This study provides the first data for the further development of pediatric enteroids as an in vitro model to study age-related variation in drug transport. Overall, drug transport in enteroids was in line with data obtained from ex vivo tissue (using chamber) experiments. Additionally, pathway analysis showed similar PK-related differences between neonates and adults in both tissue and enteroid monolayers. Given the challenge to elucidate the effect of developmental changes in the pediatric age range in human tissue, intestinal enteroids derived from pediatric patients could provide a versatile experimental platform to study pediatric phenotypes.
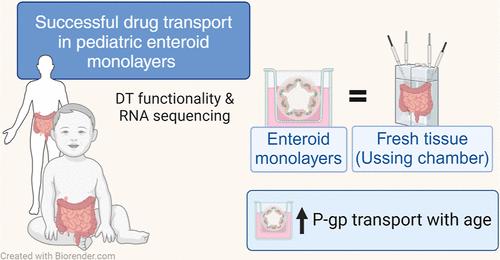
研究小儿肠道药物转运的肠道药物
出生后肠道的成熟变化会影响药物的药代动力学(PK),从而对药物的安全性和有效性产生重大影响。然而,人们对肠道中与本体相关的 PK 模式知之甚少。为了探索人类肠道单体在研究小儿肠道药物转运方面的准确性,我们比较了小儿和成人肠道单体及组织中药物转运体的功能和表达。我们培养了 14 名小儿[中位年龄(范围):44 周(2 天-13 岁)]和 5 名成人供体的肠道单体,并在其中进行了双向药物转运实验。同时,我们使用 11 个小儿组织[中位年龄(范围):54 周(15 周-10 岁)]和 6 个成人组织在乌星室中进行了类似的组织外植体实验。依那普利拉、普萘洛尔、他利洛尔和罗伐他汀分别用于检测P-gp和乳腺癌抗性蛋白(BCRP)介导的旁细胞、跨细胞和转运体外流。此外,我们还利用 RNA 测序技术比较了小儿和成人肠道单体与组织中 ADME 相关基因的表达模式。P-gp和BCRP的外排转运在肠道和组织中具有可比性。与乌星室相比,P-gp 和 BCRP 在肠道单层中对他利洛尔和罗伐他汀的外排率(ER)更高,这可能是由于模型设置和存在的细胞层的实验差异造成的。对与年龄相关性的探索性统计显示,在肠道单层中,P-gp 的ER随年龄呈上升趋势,但并不显著。在乌星室设置中,观察到依那普利拉和普萘洛尔的转运随年龄的增长而降低。重要的是,RNA 测序通路分析表明,新生儿和成人之间药物代谢的年龄相关性差异存在于肠道和肠道组织中。在组织和肠道单层中也观察到了 0 至 6 个月大的新生儿与成人之间与年龄有关的差异,但程度较小。这项研究为进一步开发小儿肠道作为体外模型来研究药物转运中与年龄相关的变化提供了第一手数据。总体而言,药物在肠道内的转运与体内外组织(使用腔室)实验所获得的数据一致。此外,路径分析显示新生儿和成人在组织和肠道单层中存在类似的 PK 相关差异。鉴于在人体组织中阐明儿科年龄段发育变化的影响是一项挑战,从儿科患者中提取的肠道内脏可以为研究儿科表型提供一个多功能的实验平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


