The Pharmacokinetics and Liver-Targeting Evaluation of Silybin Liposomes Mediated by the NTCP/OCTN2 Dual Receptors
IF 4.5
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
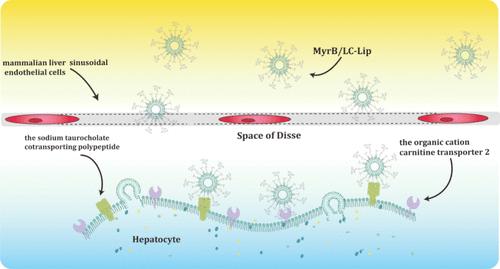
The disadvantage of a traditional dosage regimen is the inability to deliver a sufficient drug concentration to the lesion site, which can result in adverse side effects due to nonspecific drug delivery. Actively targeting hepatic cells is a promising therapeutic strategy for liver disease. In this study, l-carnitine and a targeting peptide derived from the hepatitis B virus large envelope protein were used to modify liposomes for drug delivery to the liver through the sodium taurocholate cotransporting polypeptide (NTCP) and the organic cation/carnitine transporter 2 (OCTN2) receptors. Silybin was selected as the model drug. The solubility of silybin can reach 0.3 mg/mL after encapsulation in liposomes. The NTCP-specific and OCTN2-accelerated Myrcludex B and l-carnitine dual-modified liposomes were validated in vitro. The uptake of coumarin-6 in dual ligand-modified liposomes by hepatocytes was up to 2.36 μg/mg compared with unmodified liposomes (1.05 μg/mg). The pharmacokinetics and targeting abilities of various liposome formulations were evaluated in Kunming mice. Targeted liposomes increased the concentration of silybin and prolonged the drug’s retention time in the liver. The area under the liver’s pharmacokinetic curve of targeted liposomes was twice that of silybin injection, suggesting the promising application potential of silybin-loaded hepatotropic nanovesicles.
由 NTCP/OCTN2 双受体介导的水飞蓟宾脂质体的药代动力学和肝脏靶向评估
传统给药方案的缺点是无法将足够浓度的药物输送到病变部位,这可能会由于非特异性给药而导致不良副作用。积极靶向肝细胞是一种很有前景的肝病治疗策略。本研究利用左旋肉碱和源自乙型肝炎病毒大包膜蛋白的靶向肽对脂质体进行修饰,通过牛磺胆酸钠共转运多肽(NTCP)和有机阳离子/肉碱转运体2(OCTN2)受体将药物输送到肝脏。水飞蓟宾被选为模型药物。水飞蓟宾被脂质体包裹后的溶解度可达 0.3 毫克/毫升。体外验证了NTCP特异性和OCTN2-加速的水飞蓟宾B和左旋肉碱双重修饰脂质体。与未修饰脂质体(1.05 μg/mg)相比,肝细胞对双配体修饰脂质体中香豆素-6的摄取量高达2.36 μg/mg。在昆明小鼠体内评估了各种脂质体制剂的药代动力学和靶向能力。靶向脂质体提高了水飞蓟宾的浓度,延长了药物在肝脏中的滞留时间。靶向脂质体在肝脏的药代动力学曲线下面积是水飞蓟宾注射液的两倍,这表明水飞蓟宾肝药纳米载体具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Pharmaceutics
医学-药学
CiteScore
8.00
自引率
6.10%
发文量
391
审稿时长
2 months
期刊介绍:
Molecular Pharmaceutics publishes the results of original research that contributes significantly to the molecular mechanistic understanding of drug delivery and drug delivery systems. The journal encourages contributions describing research at the interface of drug discovery and drug development.
Scientific areas within the scope of the journal include physical and pharmaceutical chemistry, biochemistry and biophysics, molecular and cellular biology, and polymer and materials science as they relate to drug and drug delivery system efficacy. Mechanistic Drug Delivery and Drug Targeting research on modulating activity and efficacy of a drug or drug product is within the scope of Molecular Pharmaceutics. Theoretical and experimental peer-reviewed research articles, communications, reviews, and perspectives are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


