(Z)-Selective Isomerization of 1,1-Disubstituted Alkenes by Scandium-Catalyzed Allylic C–H Activation
IF 3.784
3区 化学
Q1 Chemistry
引用次数: 0
Abstract
The isomerization of 1,1-disubstituted alkenes through 1,3-hydrogen shift is an atom-efficient route for synthesizing trisubstituted alkenes, which are important moieties in many natural products, pharmaceuticals, and organic materials. However, this reaction often encounters regio- and stereoselectivity challenges, typically yielding E/Z-mixtures of the alkene products or thermodynamically favored (E)-alkenes. Herein, we report the (Z)-selective isomerization of 1,1-disubstituted alkenes to trisubstituted (Z)-alkenes via the regio- and stereospecific activation of an allylic C–H bond. The key to the success of this unprecedented transformation is the use of a sterically demanding half-sandwich scandium catalyst in combination with a bulky quinoline compound, 2-tert-butylquinoline. Deuterium-labeling experiments and density functional theory (DFT) calculations have revealed that 2-tert-butylquinoline not only facilitates the C═C bond transposition through hydrogen shuttling but also governs the regio- and stereoselectivity due to the steric hindrance of the tert-butyl group. This protocol enables the synthesis of diverse (Z)-configured acyclic trisubstituted alkenes and endocyclic trisubstituted alkenes from readily accessible 1,1-disubstituted alkenes. It offers an efficient and selective route for preparing a new family of synthetically challenging (Z)-trisubstituted alkenes with broad substrate scope, 100% atom efficiency, high regio- and stereoselectivity, and an unprecedented reaction mechanism.
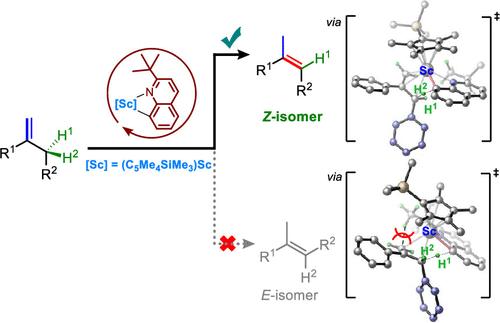
通过钪催化烯丙基 C-H 活化实现 1,1-二取代烯烃的 (Z)-选择性异构化
1,1-二取代烯通过 1,3- 氢转移进行异构化是合成三取代烯的一条原子高效路线,三取代烯是许多天然产品、药品和有机材料中的重要分子。然而,这一反应经常会遇到区域和立体选择性方面的挑战,通常会产生 E/Z 混合物的烯产物或热力学上有利的 (E) 烯。在此,我们报告了通过烯丙基 C-H 键的区域和立体选择性活化,1,1-二取代烯烃向三取代 (Z) 烯烃的 (Z) 选择性异构化。这一史无前例的转化之所以能够成功,关键在于使用了一种立体要求极高的半三明治钪催化剂,并结合了一种大体积喹啉化合物--2-叔丁基喹啉。氘标记实验和密度泛函理论(DFT)计算显示,2-叔丁基喹啉不仅通过氢穿梭促进了 C═C 键的换位,而且由于叔丁基的立体阻碍作用,还控制了区域和立体选择性。这种方法可以从容易获得的 1,1-二取代烯合成各种 (Z) 构型的无环三取代烯和内环三取代烯。它为制备具有广泛底物范围、100% 原子效率、高区域和立体选择性以及前所未有的反应机理的新型合成挑战性 (Z) 三取代烯家族提供了一条高效和选择性路线。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Combinatorial Science
CHEMISTRY, APPLIED-CHEMISTRY, MEDICINAL
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
The Journal of Combinatorial Chemistry has been relaunched as ACS Combinatorial Science under the leadership of new Editor-in-Chief M.G. Finn of The Scripps Research Institute. The journal features an expanded scope and will build upon the legacy of the Journal of Combinatorial Chemistry, a highly cited leader in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


