Adsorption of Sulfamethoxazole on Layered Double Hydroxides: Molecular Dynamics Modeling
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
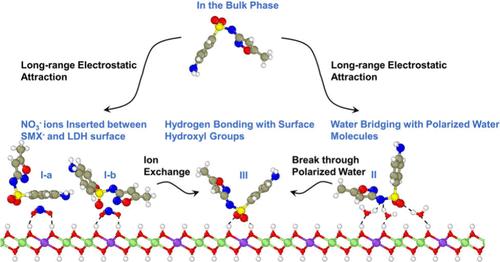
Sulfamethoxazole has been extensively detected in aquatic environments worldwide, posing a great risk to human health. Although clay minerals are effective adsorbents for contaminants, investigations of the adsorption of sulfamethoxazole on clay minerals remain limited. In this paper, the adsorption of anionic sulfamethoxazole (SMX–) from bulk to layered double hydroxides (LDH) surface was simulated by molecular dynamics. The electrostatic interaction between LDH and SMX– drove the adsorption. Four adsorption configurations of SMX– were identified: two with NO3– ions inserted between SMX– and the LDH surface, one via water bridging and one directly forming hydrogen bonding with the hydroxyl hydrogen atoms of LDH. The adsorbed SMX– exhibited significantly lower diffusion coefficient than that in the bulk, indicating that LDH has an advantage over common clay adsorbents (e.g., montmorillonite) in removing SMX– from water. The adsorption process was demonstrated to be enthalpy-driven by thermodynamic analysis. The free energy well in the weakly ordered water layer was deeper than that in the strongly ordered water layer, which explained why SMX– preferred to stay in the former. To enter the free energy barrier in the latter, SMX– was required to overcome the barrier by exchanging with adsorbed NO3– ions or breaking the ordered water layer.
磺胺甲噁唑在层状双氢氧化物上的吸附:分子动力学模型
磺胺甲噁唑在世界各地的水生环境中被广泛检测到,对人类健康构成了巨大风险。虽然粘土矿物是污染物的有效吸附剂,但有关磺胺甲噁唑在粘土矿物上吸附的研究仍然有限。本文通过分子动力学模拟了阴离子磺胺甲噁唑(SMX-)从块体到层状双氢氧化物(LDH)表面的吸附过程。LDH 与 SMX- 之间的静电作用驱动了吸附。确定了 SMX- 的四种吸附构型:两种是 NO3- 离子插入 SMX- 和 LDH 表面之间,一种是通过水桥,还有一种是直接与 LDH 的羟基氢原子形成氢键。被吸附的 SMX- 的扩散系数明显低于散体中的扩散系数,这表明 LDH 在去除水中的 SMX- 方面比普通粘土吸附剂(如蒙脱石)更有优势。热力学分析表明,吸附过程是由焓驱动的。弱有序水层中的自由能井比强有序水层中的自由能井深,这就解释了为什么 SMX- 喜欢留在前者中。要进入后者的自由能障,SMX- 需要通过与吸附的 NO3- 离子交换或破坏有序水层来克服自由能障。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


