The Alkynyl π Bond of sp-C Enhanced Rapid, Reversible Li–C Coupling to Accelerate Reaction Kinetics of Lithium Ions
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Graphdiyne (GDY) is a promising anode for rechargeable batteries with high capacity, outstanding cyclic stability, and low diffusion energy. The unique structure of GDY endows distinctive mechanisms for metal-ion storage, and it is of great significance to further visualize the complex reaction kinetics of the redox process. Here, we systematically tracked the reaction kinetics and provided mechanistic insights into the lithium ions in the GDY to reveal the feature of the cation-π effect. It has been demonstrated that, unlike only one π bond in sp2-C, π electrons provided by one of the two alkynyl π bonds in sp-C can achieve proper interaction and speedy capture of lithium ions; thus, reversible Li–C coupling can be formed between electron-rich sp-C and lithium ions. In addition to interlayer intercalation in sp2-C regions, nanopores filling triangular-like cavities composed of highly conjugated sp-C contribute to the major capacity in flat voltage plateau regions. Therefore, a capture/pores filling-intercalation hybrid mechanism can be found in GDY. The coexistence of sp and sp2 carbon enables GDY electrodes with rapid Li+ diffusion, high capacity of over 1435 mAh g–1, extraordinary rate capability, and cyclic stability for more than 10000 cycles at 10A g–1. These results provide guidance for developing advanced carbon electrodes with optimized reaction kinetics for rechargeable batteries.
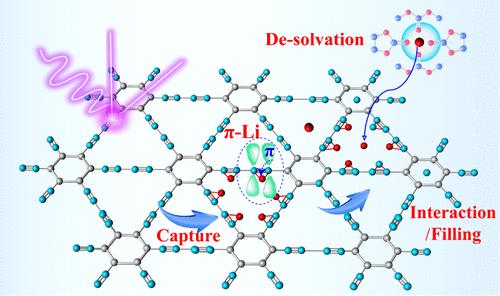
sp-C 的炔基π键增强了快速、可逆的锂-碳耦合,从而加快了锂离子的反应动力学
Graphdiyne(GDY)具有高容量、出色的循环稳定性和低扩散能,是一种很有前途的充电电池阳极。GDY 的独特结构赋予了其独特的金属离子存储机制,进一步观察氧化还原过程中复杂的反应动力学具有重要意义。在此,我们对反应动力学进行了系统追踪,并对 GDY 中的锂离子进行了机理研究,揭示了阳离子-π效应的特征。研究表明,与 sp2-C 中只有一个π键不同,sp-C 中两个炔基π键中的一个所提供的π电子可以实现适当的相互作用并迅速捕获锂离子;因此,富电子的 sp-C 与锂离子之间可以形成可逆的锂-C 耦合。除了 sp2-C 区域的层间插层外,由高度共轭 sp-C 构成的三角形空腔中的纳米孔也是平电压高原区的主要容量来源。因此,在 GDY 中可以发现一种捕获/孔填充-叠加混合机制。sp 和 sp2 碳的共存使 GDY 电极具有快速的 Li+ 扩散、超过 1435 mAh g-1 的高容量、非凡的速率能力以及在 10A g-1 下超过 10000 次循环的循环稳定性。这些结果为开发具有优化反应动力学的先进碳电极提供了指导,可用于充电电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


