Remote Functionalization of Inert C(sp3)–H Bonds via Dual Catalysis Driven by Alkene Hydrofluoroalkylation Using Industrial Feedstocks
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We have developed a dual-catalytic system capable of site-selective azidation of inert C(sp3)–H bonds with concomitant and modular anti-Markovnikov alkene fluoroalkylation. The protocol leverages the synergetic cooperation of both the photocatalyst and earth-abundant iron catalyst to deliver two radical species in succession to minimally functionalized alkenes. This powerful catalyst system exhibits broad scope, mild conditions, and excellent regioselectivity for a variety of substrates and fluoroalkyl fragments. The key to this C-centered radical relay is the matched rate of both photocatalytic and iron catalytic cycles, ensuring selective azidofluoroalkylation with a broad array of fluoroalkyl sources from trivial reagents.
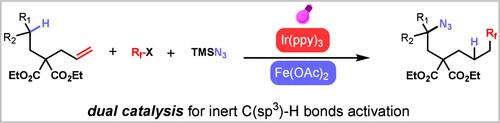
利用工业原料,通过烯烃氢氟烷基化驱动双催化技术实现惰性 C(sp3)-H 键的远程官能化
我们开发了一种双催化系统,能够对惰性 C(sp3)-H 键进行位点选择性叠氮化,同时进行模块化反马尔科夫尼科夫烯烃氟烷基化反应。该方案利用光催化剂和富土铁催化剂的协同作用,将两种自由基依次传递给最小官能化的烯烃。这种功能强大的催化剂体系适用范围广、条件温和,对多种底物和氟烷基片段具有优异的区域选择性。这种以 C 为中心的自由基中继的关键在于光催化和铁催化循环的匹配速率,从而确保了利用微不足道的试剂对各种氟烷基进行选择性叠氮氟烷基化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


