Culture system for longitudinal monitoring of bone dynamics ex vivo
IF 3.5
2区 生物学
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
To quantify and visualize both bone formation and resorption within osteochondral explants cultured ex vivo is challenging with the current analysis techniques. An approach that enables monitoring of bone remodeling dynamics is longitudinal microcomputed tomography (µCT), a non-destructive technique that relies on repeated µCT scanning and subsequent registration of consecutive scans. In this study, a two-compartment culture system suitable for osteochondral explants that allowed for µCT scanning during ex vivo culture was established. Explants were scanned repeatedly in a fixed orientation, which allowed assessment of bone remodeling due to adequate image registration. Using this method, bone formation was found to be restricted to the outer surfaces when cultured statically. To demonstrate that the culture system could capture differences in bone remodeling, explants were cultured statically and under dynamic compression as loading promotes osteogenesis. No quantitative differences between static and dynamic culture were revealed. Still, only in dynamic conditions, bone formation was visualized on trabecular surfaces located within the inner cores, suggesting enhanced bone formation towards the center of the explants upon mechanical loading. Taken together, the ex vivo culture system in combination with longitudinal µCT scanning and subsequent registration of images demonstrated potential for evaluating bone remodeling within explants.
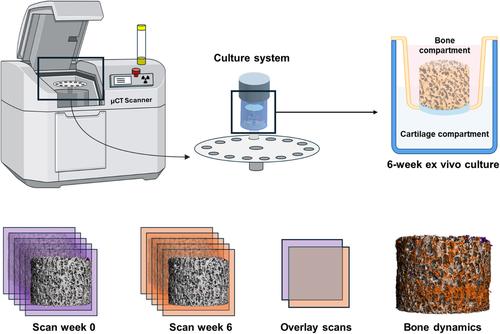
用于纵向监测体内外骨骼动态的培养系统
要量化和可视化体外培养骨软骨外植体的骨形成和骨吸收,目前的分析技术还很难做到。纵向微计算机断层扫描(µCT)是一种能够监测骨重塑动态的方法,它是一种非破坏性技术,依赖于µCT的重复扫描和连续扫描的后续注册。本研究建立了一种适合骨软骨外植体的两室培养系统,可在体外培养过程中进行 µCT 扫描。外植体以固定的方向反复扫描,通过适当的图像配准可以评估骨重塑情况。使用这种方法发现,在静态培养时,骨形成仅限于外表面。为了证明该培养系统能捕捉到骨重塑的差异,对外植体进行了静态培养和动态压缩培养,因为加载会促进骨生成。结果显示,静态培养和动态培养之间没有数量上的差异。不过,只有在动态条件下,位于内核的骨小梁表面才能看到骨形成,这表明在机械加载时,外植体中心的骨形成会增强。综上所述,体内外培养系统与纵向 µCT 扫描及随后的图像配准相结合,证明了评估外植体内骨重塑的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biotechnology and Bioengineering
工程技术-生物工程与应用微生物
CiteScore
7.90
自引率
5.30%
发文量
280
审稿时长
2.1 months
期刊介绍:
Biotechnology & Bioengineering publishes Perspectives, Articles, Reviews, Mini-Reviews, and Communications to the Editor that embrace all aspects of biotechnology. These include:
-Enzyme systems and their applications, including enzyme reactors, purification, and applied aspects of protein engineering
-Animal-cell biotechnology, including media development
-Applied aspects of cellular physiology, metabolism, and energetics
-Biocatalysis and applied enzymology, including enzyme reactors, protein engineering, and nanobiotechnology
-Biothermodynamics
-Biofuels, including biomass and renewable resource engineering
-Biomaterials, including delivery systems and materials for tissue engineering
-Bioprocess engineering, including kinetics and modeling of biological systems, transport phenomena in bioreactors, bioreactor design, monitoring, and control
-Biosensors and instrumentation
-Computational and systems biology, including bioinformatics and genomic/proteomic studies
-Environmental biotechnology, including biofilms, algal systems, and bioremediation
-Metabolic and cellular engineering
-Plant-cell biotechnology
-Spectroscopic and other analytical techniques for biotechnological applications
-Synthetic biology
-Tissue engineering, stem-cell bioengineering, regenerative medicine, gene therapy and delivery systems
The editors will consider papers for publication based on novelty, their immediate or future impact on biotechnological processes, and their contribution to the advancement of biochemical engineering science. Submission of papers dealing with routine aspects of bioprocessing, description of established equipment, and routine applications of established methodologies (e.g., control strategies, modeling, experimental methods) is discouraged. Theoretical papers will be judged based on the novelty of the approach and their potential impact, or on their novel capability to predict and elucidate experimental observations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


