Electrocatalytic Aromatic Alcohols Splitting to Aldehydes and H2 Gas
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Selective electrocatalytic transformation of alcohols to aldehydes offers an efficient and environmentally friendly platform for the simultaneous production of fine chemicals and pure hydrogen gas. However, traditional alcohol oxidation reactions (AORs) in aqueous electrolyte unavoidably face competitive reactions (e.g., water oxidation and overoxidations reactions) for the presence of active oxygen species from water oxidation, causing an unwanted decrease in final efficiency and selectivity. Here, we developed an integrated all-solid proton generator-transfer electrolyzer to trigger the pure alcohol splitting reaction (ASR). In this splitting process, only O–H and C–H bonds can be cleaved at the proton generator (Pt nanoparticles), thereby completely avoiding all competitive reactions involving oxygen active species to give a > 99% selectivity to aldehydes. The as-generated protons are transported to the cathode by a three-dimensional (3D) conducting network (assemblies of ionomers and carbon spheres) for efficient hydrogen production. Unlike the poor selectivity (<22%) and durability (<3 h) of a conventional AOR electrolyzer, this ASR electrolyzer could be continuously operated at a low cell voltage of 1.2 V for at least 10 days to give a high Faradaic efficiency of 80–93% for aldehyde production.
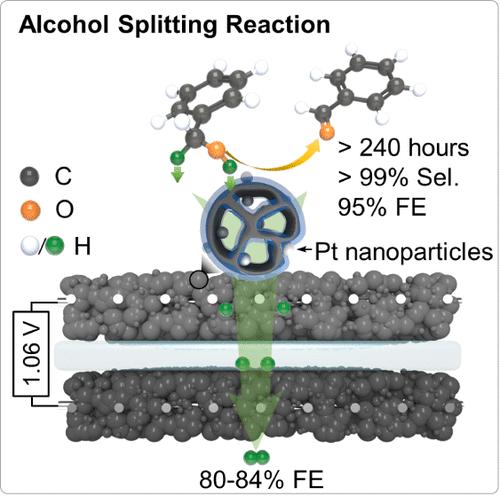
选择性电催化将醇类转化为醛类为同时生产精细化学品和纯氢气提供了一个高效、环保的平台。然而,在水电解质中进行的传统醇氧化反应(AORs)不可避免地会因水氧化产生的活性氧的存在而面临竞争反应(如水氧化和过氧化反应),导致最终效率和选择性下降。在此,我们开发了一种集成的全固态质子发生器-转移电解槽,用于引发纯酒精裂解反应(ASR)。在这一分裂过程中,只有 O-H 和 C-H 键可以在质子发生器(铂纳米粒子)上裂解,从而完全避免了所有涉及氧活性物种的竞争反应,使对醛的选择性达到 99%。生成的质子通过三维(3D)导电网络(离子聚合物和碳球的集合体)传输到阴极,从而高效制氢。与传统 AOR 电解槽的低选择性(22%)和低耐久性(3 小时)不同,这种 ASR 电解槽可以在 1.2 V 的低电池电压下连续运行至少 10 天,从而获得 80-93% 的高 Faradaic 效率来生产醛。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


