Modulating Decarboxylative Oxidation Photocatalysis by Ligand Engineering of Atomically Precise Copper Nanoclusters
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Copper nanoclusters (Cu NCs) characterized by their well-defined electronic and optical properties are an ideal platform for organic photocatalysis and exploring atomic-level behaviors. However, their potential as greener, efficient catalysts for challenging reactions like decarboxylative oxygenation under mild conditions remains unexplored. Herein, we present Cu13(Nap)3(PPh3)7H10 (hereafter Cu13Nap), protected by 1-naphthalene thiolate (Nap), which performs well in decarboxylative oxidation (90% yield) under photochemical conditions. In comparison, the isostructural Cu13(DCBT)3(PPh3)7H10 (hereafter Cu13DCBT), stabilized by 2,4-dichlorobenzenethiolate (DCBT), yields only 28%, and other previously reported Cu NCs (Cu28, Cu29, Cu45, Cu57, and Cu61) yield in the range of 6–18%. The introduction of naphthalene thiolate to the surface of Cu13 NCs influences their electronic structure and charge transfer in the ligand shell, enhancing visible light absorption and catalytic performance. Density functional theory (DFT) and experimental evidence suggest that the reaction proceeds primarily through an energy transfer mechanism. The energy transfer pathway is uncommon in the context of previous reports for decarboxylative oxidation reactions. Our findings suggest that strategically manipulating ligands holds significant potential for creating composite active sites on atomically precise copper NCs, resulting in enhanced catalytic efficacy and selectivity across various challenging reactions.
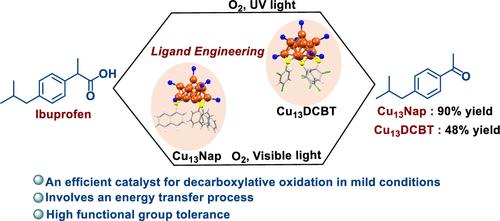
通过原子精度铜纳米团簇配体工程调控脱羧氧化光催化作用
纳米铜簇(Cu NCs)具有明确的电子和光学特性,是有机光催化和探索原子级行为的理想平台。然而,它们作为在温和条件下进行脱羧加氧等挑战性反应的更环保、更高效催化剂的潜力仍有待开发。在此,我们介绍了受 1-萘硫酸盐(Nap)保护的 Cu13(Nap)3(PPh3)7H10(以下简称 Cu13Nap),它在光化学条件下的脱羧氧化反应中表现出色(产率达 90%)。相比之下,由 2,4-二氯苯硫酚(DCBT)稳定的等结构 Cu13(DCBT)3(PPh3)7H10(以下简称 Cu13DCBT)的产率仅为 28%,而之前报道的其他 Cu NCs(Cu28、Cu29、Cu45、Cu57 和 Cu61)的产率在 6-18% 之间。在 Cu13 NCs 表面引入萘硫酸盐会影响其电子结构和配体外壳中的电荷转移,从而增强可见光吸收和催化性能。密度泛函理论(DFT)和实验证据表明,该反应主要通过能量传递机制进行。在以往有关脱羧氧化反应的报道中,能量转移途径并不常见。我们的研究结果表明,对配体进行策略性操作具有在原子精确的铜 NC 上创建复合活性位点的巨大潜力,从而在各种具有挑战性的反应中提高催化效率和选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


