Regulatory responses and approval status of artificial intelligence medical devices with a focus on China
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
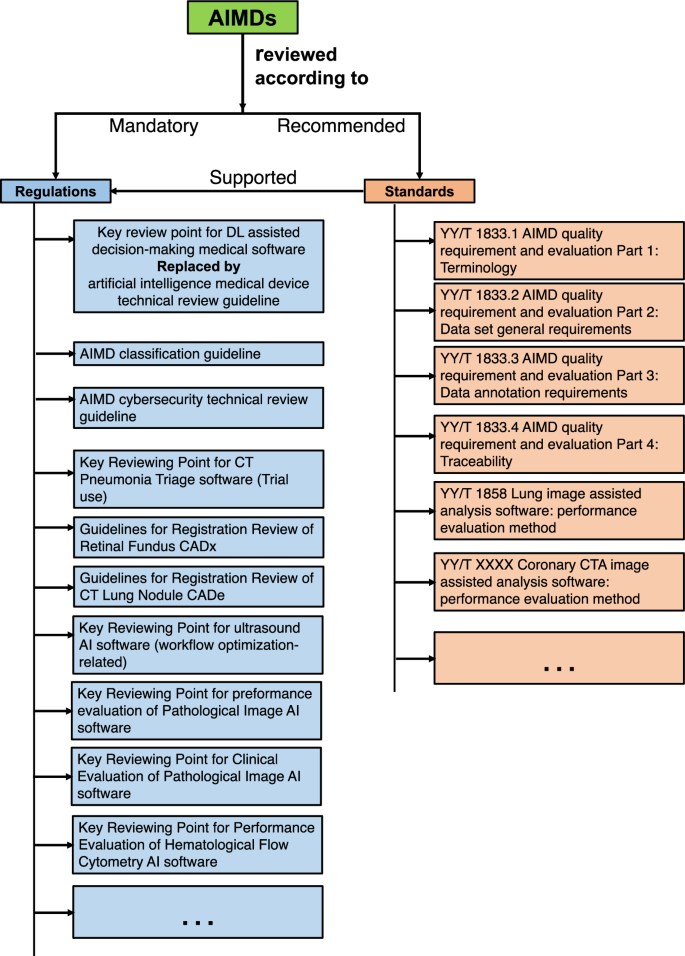
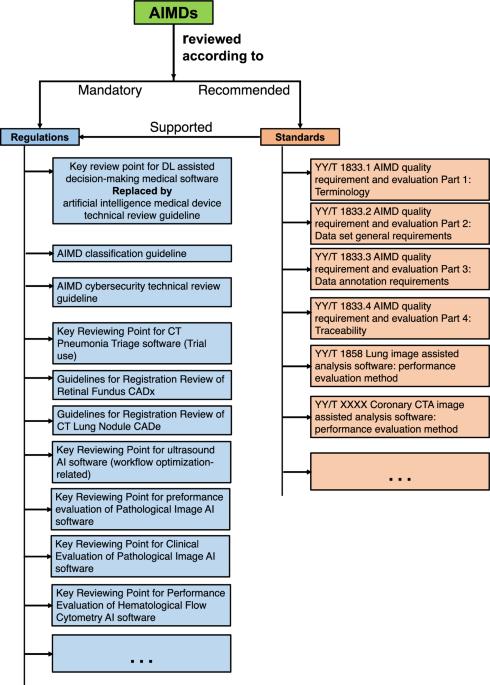
This paper focuses on how regulatory bodies respond to artificial intelligence (AI)-enabled medical devices. To achieve this, we present a comparative overview of the United States (USA), European Union (EU), and China. Our search in the governmental database identified 59 AI medical devices approved in China as of July 2023. In comparison to the rules-based regulatory approach in China, the approaches in the USA and EU are more standards-oriented.
以中国为重点的人工智能医疗器械监管对策和审批情况
本文重点探讨监管机构如何应对人工智能(AI)医疗设备。为此,我们对美国(USA)、欧盟(EU)和中国进行了比较概述。我们在政府数据库中搜索到,截至 2023 年 7 月,中国共批准了 59 种人工智能医疗设备。与中国以规则为基础的监管方法相比,美国和欧盟的方法更加以标准为导向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


