ARGX-119 is an agonist antibody for human MuSK that reverses disease relapse in a mouse model of congenital myasthenic syndrome
IF 15.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
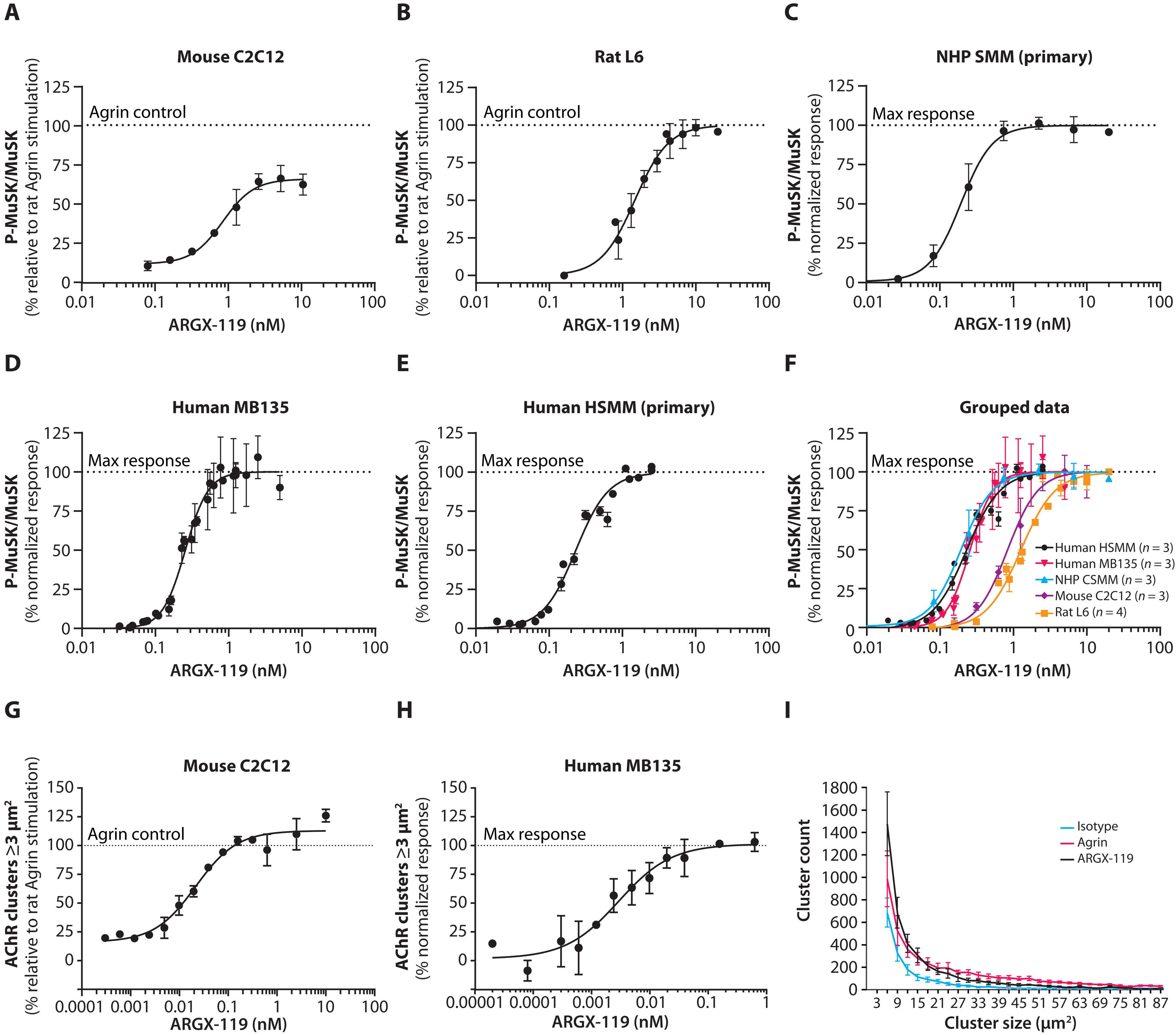
Muscle-specific kinase (MuSK) is essential for the formation, function, and preservation of neuromuscular synapses. Activation of MuSK by a MuSK agonist antibody may stabilize or improve the function of the neuromuscular junction (NMJ) in patients with disorders of the NMJ, such as congenital myasthenia (CM). Here, we generated and characterized ARGX-119, a first-in-class humanized agonist monoclonal antibody specific for MuSK, that is being developed for treatment of patients with neuromuscular diseases. We performed in vitro ligand-binding assays to show that ARGX-119 binds with high affinity to the Frizzled-like domain of human, nonhuman primate, rat, and mouse MuSK, without off-target binding, making it suitable for clinical development. Within the Fc region, ARGX-119 harbors L234A and L235A mutations to diminish potential immune-activating effector functions. Its mode of action is to activate MuSK, without interfering with its natural ligand neural Agrin, and cluster acetylcholine receptors in a dose-dependent manner, thereby stabilizing neuromuscular function. In a mouse model of DOK7 CM, ARGX-119 prevented early postnatal lethality and reversed disease relapse in adult Dok7 CM mice by restoring neuromuscular function and reducing muscle weakness and fatigability in a dose-dependent manner. Pharmacokinetic studies in nonhuman primates, rats, and mice revealed a nonlinear PK behavior of ARGX-119, indicative of target-mediated drug disposition and in vivo target engagement. On the basis of this proof-of-concept study, ARGX-119 has the potential to alleviate neuromuscular diseases hallmarked by impaired neuromuscular synaptic function, warranting further clinical development.
ARGX-119 是一种人 MuSK 激动剂抗体,可逆转先天性肌无力综合征小鼠模型的疾病复发
肌肉特异性激酶(MuSK)对神经肌肉突触的形成、功能和保存至关重要。通过 MuSK 激动剂抗体激活 MuSK 可稳定或改善神经肌肉接头(NMJ)功能,如先天性肌萎缩症(CM)患者。在这里,我们生成并鉴定了 ARGX-119,这是一种特异于 MuSK 的首类人源化激动剂单克隆抗体,目前正在开发用于治疗神经肌肉疾病患者。我们进行了体外配体结合试验,结果表明 ARGX-119 能与人类、非人灵长类动物、大鼠和小鼠 MuSK 的 Frizzled 样结构域高亲和力结合,且无脱靶结合,因此适合临床开发。在 Fc 区域,ARGX-119 存在 L234A 和 L235A 突变,从而削弱了潜在的免疫激活效应功能。它的作用模式是激活 MuSK,而不干扰其天然配体 Agrin,并以剂量依赖性方式集聚乙酰胆碱受体,从而稳定神经肌肉功能。在一种 DOK7 CM 小鼠模型中,ARGX-119 通过恢复神经肌肉功能并以剂量依赖性方式减少肌无力和疲劳,防止了出生后早期致死,并逆转了成年 Dok7 CM 小鼠的疾病复发。在非人灵长类动物、大鼠和小鼠体内进行的药代动力学研究显示,ARGX-119 的 PK 行为是非线性的,表明了靶点介导的药物处置和体内靶点参与。在这项概念验证研究的基础上,ARGX-119 有可能缓解以神经肌肉突触功能受损为特征的神经肌肉疾病,值得进一步临床开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


