Pulmonary complications post allogeneic haematopoietic stem cell transplant in children
Abstract
Objectives
Haematopoietic stem cell transplant (HCT) is a cellular therapy that, whilst curative for a child's underlying disease, carries significant risk of mortality, including because of pulmonary complications. The aims of this study were to describe the burden of pulmonary complications post-HCT in a cohort of Australian children and identify risk factors for the development of these complications.
Methods
Patients were identified from the HCT databases at two paediatric transplant centres in Australia. Medical records were reviewed, and demographics, HCT characteristics and pulmonary complications documented. Relative risk ratio was used to identify risk factors for developing pulmonary complications prior to first transplant episode, and survival analysis performed to determine hazard ratio.
Results
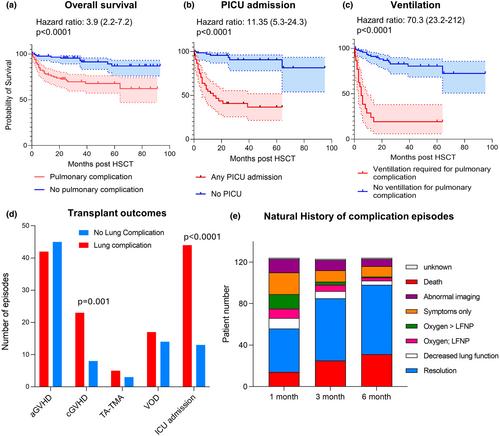
In total, 243 children underwent transplant during the study period, and pulmonary complications occurred in 48% (117/243) of children. Infectious complications were more common (55%) than non-infective complications (18%) and 26% of patients developed both. Risk factors for the development of pulmonary complications included the following: diagnoses of MPAL (RR 2.16, P = 0.02), matched unrelated donor (RR1.34, P = 0.03), peripheral blood (RR 1.36, P = 0.028) or cord blood (RR 1.73, P = 0.012) as the stem cell source and pre-existing lung disease (RR1.72, P < 0.0001). Children with a post-HCT lung complication had a significantly increased risk of mortality compared with those who did not (HR 3.9, P < 0.0001).
Conclusion
This study demonstrates pulmonary complications continue to occur frequently in children post-HCT and contribute significantly to mortality. Highlighting the need for improved strategies to identify patients at risk pre-transplant and enhanced treatments for those who develop lung disease.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


