Structure elucidation, absolute configuration, and biological evaluation of cyclic peroxides from the sponge Plakinastrella sp.
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Two cyclic peroxides, plakortides V (1) and W (2) were purified from the organic extract of the sponge Plakinastrella sp. Their planar structures were established based on extensive NMR and MS analysis and the absolute configurations of the three stereogenic centers of the 1,2-dioxane moiety were determined to be 3R,4S,6S by comparative analysis of the 1H NMR spectral data of the R- or S-MTPA Mosher esters. Compounds 1 and 2 exhibited potent cytotoxic activity against LOX IMVI (melanoma), UO-31 (renal), and HL-60 (TB) (leukemia) cell lines in the NCI-60 cytotoxicity assay.
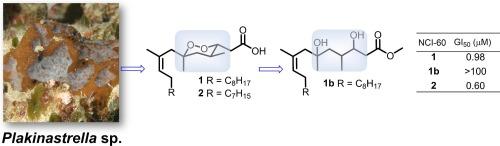
海绵 Plakinastrella sp.环过氧化物的结构阐释、绝对构型和生物学评价
从海绵 Plakinastrella sp.的有机提取物中纯化出了两种环过氧化物,即 plakortides V (1) 和 W (2)。通过对 R- 或 S-MTPA Mosher 酯的 1H NMR 光谱数据进行比较分析,确定了 1,2-二恶烷分子的三个立体中心的绝对构型为 3R,4S,6S。在 NCI-60 细胞毒性试验中,化合物 1 和 2 对 LOX IMVI(黑色素瘤)、UO-31(肾癌)和 HL-60 (TB)(白血病)细胞株具有很强的细胞毒性活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


