Rechargeable Mg–Br2 Battery with Ultrafast Bromine Chemistry
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
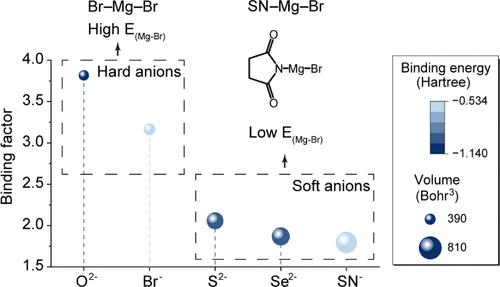
A sustainable society necessitates the support of diversified energy storage systems. Magnesium metal batteries, known for the environmental friendliness, safety of dendrite-less, cost-effective, and high volumetric capacity of magnesium metal, exhibit promising prospects. However, the high charge density of the magnesium ion leads to sluggish ion diffusion in cathodes, posing challenges for developing magnesium metal battery systems with high power and high energy density. Here, inspired by the Hard–Soft-Acid–Base theory, we propose a soft-anion-induced bond weakening strategy to address the diffusion difficulty. The bulky and broadly electron-distributed succinimide ion (SN–) in SN–Mg–Br significantly weakens the Mg–Br bond, promoting rapid magnesium ion transport and enabling ultrafast bromine chemistry, thus realizing a highly rechargeable Mg–Br2 battery prototype. Benefiting from the solubilization of SN–, the Mg–Br2 batteries achieve a high discharge plateau of 2.7 V, a remarkable specific capacity of 326 mAh gBr–1, and an impressive lifespan of 400 cycles. Attributed to the half–half diffusion/adsorption–desorption control process mechanism, the batteries can be well cycled under high-rate charging at 10 C and ultralow temperatures down to −55 °C. This bond weakening strategy may stimulate the development of battery systems with similar high charge density to magnesium ion, toward high power and high energy density, paving the way for sustainable energy storage systems.
采用超快溴化学反应的可充电 Mg-Br2 电池
可持续发展的社会需要多样化的能源储存系统的支持。镁金属电池以环保、无树枝状晶粒的安全性、高性价比和高容量而著称,前景广阔。然而,镁离子的高电荷密度导致阴极中的离子扩散缓慢,给开发高功率和高能量密度的镁金属电池系统带来了挑战。在硬-软-酸-碱理论的启发下,我们提出了一种软离子诱导的键弱化策略来解决扩散难题。SN-Mg-Br 中体积庞大、电子分布广泛的琥珀酰亚胺离子(SN-)能显著削弱 Mg-Br 键,促进镁离子的快速传输,实现超快溴化学反应,从而实现高度可充电的 Mg-Br2 电池原型。得益于 SN- 的增溶作用,Mg-Br2 电池实现了 2.7 V 的高放电平台,比容量高达 326 mAh gBr-1,使用寿命长达 400 次。由于采用了半扩散/吸附-解吸控制过程机制,电池可在 10 ℃ 的高速充电和低至 -55 ℃ 的超低温条件下循环使用。这种键弱化策略可促进开发与镁离子类似的高电荷密度、高功率和高能量密度的电池系统,为可持续储能系统铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


