Regiocontrollable B(2/3)–H Alkenylation of nido-Carboranes
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Anionic nido-carboranes, as open-cage analogues of closo-carboranes with strong hydrophilicity and higher potential in the development of biomedicines, have been notably more challenging because of their strong interaction with transition metals. While the exo-cage B–H activation reactions of nido-carboranes have been widely studied, there are few reports on the direct functionalization of B–H bonds located on a closed polyhedral sphere. Here, we report an efficient palladium-catalyzed regioselective B(2/3)–H alkenylation of nido-carboranes with various alkenes and alkyne coupling partners, enabled by 3-methylpyridine directing groups, to achieve a regiocontrollable functionalization of B(2/3)–H vertices over highly reactive exo-cage B11–H vertex in nido-carboranes.
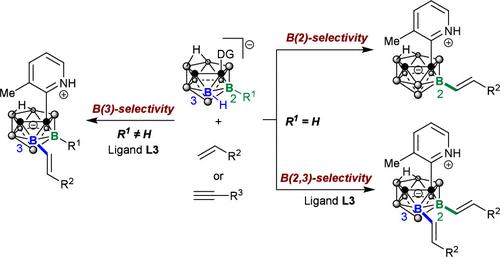
硝基硼烷的可控 B(2/3)-H 烯化反应
阴离子nido-硼烷是closedo-硼烷的开笼类似物,具有很强的亲水性,在生物医药开发方面具有更大的潜力。虽然人们已经广泛研究了尼多硼烷的外笼 B-H 活化反应,但有关位于封闭多面体上的 B-H 键直接官能化的报道却很少。在此,我们报告了在 3-甲基吡啶指导基团的作用下,通过钯催化,实现了新硼烷与各种烯烃和炔烃偶联伙伴的高效区域选择性 B(2/3)-H 烯化反应,从而在新硼烷的高活性外笼 B11-H 顶点上实现了 B(2/3)-H 顶点的区域可控官能化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


