Organocatalytic Asymmetric Tandem Reaction of Indene-Based Dienes for the Synthesis of Enantioenriched Spirodihydrofluorenes and Fluorenylamine-ketoximes Containing Axial Elements
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient asymmetric tandem reaction of indene-based dienes with isoxazole-5-ones catalyzed by chiral cinchona alkaloid-derived thioureas has been established, constructing a diverse array of novel enantioenriched spirodihydrofluorene compounds bearing isoxazolone moieties. More importantly, the spirodihydrofluorene frameworks can be effortlessly transformed into an atropisomeric fluorene skeleton bearing oxime groups. This work not only addresses the challenges in the construction of enantioenriched spirodihydrofluorene frameworks but also provides a powerful strategy for structurally novel fluorenylamine-ketoximes containing axial elements.
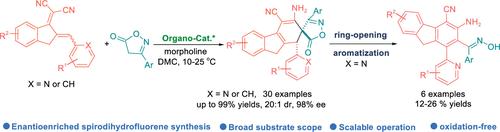
茚基二烯经有机催化不对称串联反应合成含有轴向元素的对映体螺二氢芴和芴胺酮肟
在手性金鸡纳生物碱衍生硫脲的催化下,茚基二烯烃与异噁唑-5-酮发生了高效的不对称串联反应,从而构建了一系列新颖的带有异噁唑酮分子的对映体丰富的螺二氢芴化合物。更重要的是,螺二氢芴框架可以毫不费力地转化为带有肟基团的异构芴骨架。这项工作不仅解决了构建对映体丰富的螺二氢芴框架的难题,还为含有轴向元素的结构新颖的芴胺酮肟提供了一种强有力的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


