Well-defined chiral dinuclear copper-catalyzed tandem asymmetric propargylic amination–carboxylative cyclization sequence toward chiral 2-oxazolidinone derivatives†
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a novel strategy for the synthesis of chiral 2-oxazolidinones via a dinuclear copper-catalyzed asymmetric propargylic amination–carboxylative cyclization sequence of propargylic esters with nucleophilic alkyl amines under ambient pressure of carbon dioxide. A variety of chiral 2-oxazolidinones featuring an exocyclic methylene moiety was obtained in good yields with high enantioselectivities via a one-pot operation.
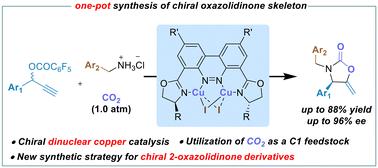
定义明确的手性双核铜催化串联不对称原炔胺化-羧基环化顺序制备手性 2-噁唑烷酮衍生物
我们报告了一种在二氧化碳环境压力下,通过双核铜催化丙炔酯与亲核烷基胺的不对称丙炔胺化-羧基环化顺序合成手性 2-噁唑烷酮的新策略。通过一锅操作,获得了多种具有外环亚甲基的手性 2-噁唑烷酮,产量高,对映选择性好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


