Development of a Deactivation-Resistant Dialkylbiarylphosphine Ligand for Pd-Catalyzed Arylation of Secondary Amines
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
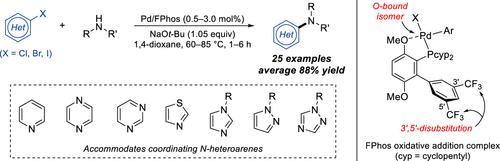
Despite the prevalence of N-heteroarenes in small-molecule pharmaceuticals, Pd-catalyzed C–N cross-coupling reactions of aryl halides and amines containing these rings remain challenging due to their ability to displace the supporting ligand via coordination to the metal center. To address this limitation, we report the development of a highly robust Pd catalyst supported by a new dialkylbiarylphosphine ligand, FPhos. The FPhos-supported catalyst effectively resists N-heteroarene-mediated catalyst deactivation to readily promote C–N coupling between a wide variety of Lewis-basic aryl halides and secondary amines, including densely functionalized pharmaceuticals. Mechanistic and structural investigations, as well as principal component analysis and density functional theory, elucidated two key design features that enable FPhos to overcome the limitations of previous ligands. First, the ligated Pd complex is stabilized through its conformational preference for the O-bound isomer, which likely resists coordination by N-heteroarenes. Second, 3′,5′-disubstitution on the non-phosphorus-containing ring of FPhos creates the ideal steric environment around the Pd center, which facilitates binding by larger secondary amines while mitigating the formation of off-cycle palladacycle species.
为钯催化仲胺芳基化开发抗失活二烷基芳基膦配体
尽管 N-杂环烯类在小分子药物中非常普遍,但由于它们能够通过与金属中心配位来置换支持配体,因此 Pd 催化的含有这些环的芳基卤化物和胺的 C-N 交叉偶联反应仍然具有挑战性。为了解决这一限制,我们报告了一种由新型二烷基芳基膦配体 FPhos 支持的高稳定性钯催化剂的开发情况。FPhos 支持的催化剂能有效抵御 N- 腙介导的催化剂失活,从而轻松促进多种路易斯碱性芳基卤化物和仲胺(包括高官能度药物)之间的 C-N 偶联。机理和结构研究以及主成分分析和密度泛函理论阐明了 FPhos 的两个关键设计特征,使其能够克服以往配体的局限性。首先,配位的钯复合物通过其构象对 O-结合异构体的偏好而趋于稳定,而 O-结合异构体可能会抵制 N-异戊二烯的配位。其次,FPhos 的非含磷环上的 3′,5′-二取代作用在钯中心周围创造了理想的立体环境,这有利于与较大的仲胺结合,同时减少了偏离周期的钯循环物种的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


