A drug mix and dose decision algorithm for individualized type 2 diabetes management
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
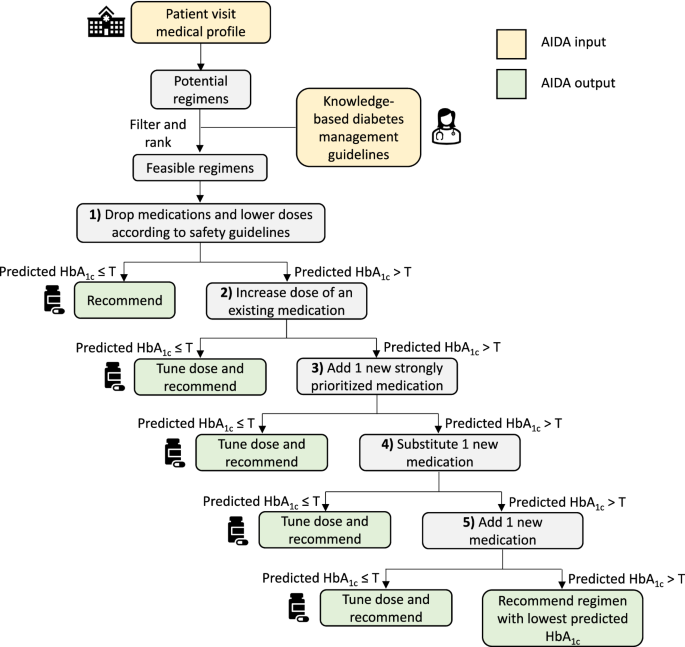
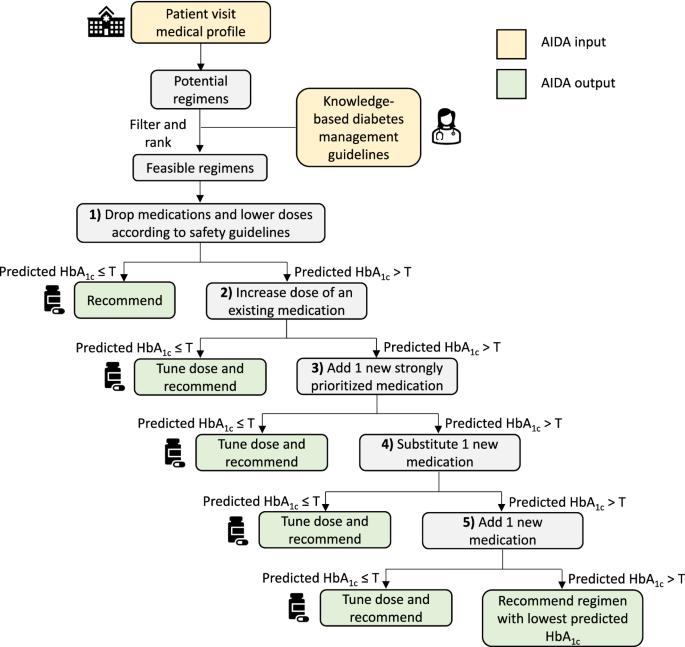
Pharmacotherapy guidelines for type 2 diabetes (T2D) emphasize patient-centered care, but applying this approach effectively in outpatient practice remains challenging. Data-driven treatment optimization approaches could enhance individualized T2D management, but current approaches cannot account for drug-specific and dose-dependent variations in safety and efficacy. We developed and evaluated an AI Drug mix and dose Advisor (AIDA) for glycemic management, using electronic medical records from 107,854 T2D patients in the SingHealth Diabetes Registry. Given a patient’s medical profile, AIDA leverages a predict-then-optimize approach to identify the minimal drug mix and dose changes required to optimize glycemic control, subject to clinical knowledge-based guidelines. On unseen data from large internal, external, and temporal validation sets, AIDA recommendations were estimated to improve post-visit glycated hemoglobin (HbA1c) by an average of 0.40–0.68% over standard of care (P < 0.0001). In qualitative evaluations on 60 diverse cases by a panel of three endocrinologists, AIDA recommendations were mostly rated as reasonable and precise. Finally, AIDA’s ability to account for drug-dose specifics offered several advantages over competing methods, including greater consistency with practice preferences and clinical guidelines for practical but effective options, indication-based treatments, and renal dosing. As AIDA provides drug-dose recommendations to improve outcomes for individual T2D patients, it could be used for clinical decision support at point-of-care, especially in resource-limited settings.
个性化 2 型糖尿病管理的药物组合和剂量决策算法
2 型糖尿病(T2D)的药物治疗指南强调以患者为中心的护理,但在门诊实践中有效应用这种方法仍具有挑战性。以数据为驱动的治疗优化方法可以加强 2 型糖尿病的个体化管理,但目前的方法无法解释药物在安全性和有效性方面的特异性和剂量依赖性变化。我们利用新加坡保健集团糖尿病登记处 107,854 名 T2D 患者的电子病历,开发并评估了用于血糖管理的人工智能药物组合和剂量顾问(AIDA)。根据患者的医疗档案,AIDA 采用预测-优化方法,根据基于临床知识的指南,确定优化血糖控制所需的最小药物组合和剂量变化。在来自大型内部、外部和时间验证集的未见数据中,AIDA 的建议估计可将就诊后的糖化血红蛋白 (HbA1c) 平均提高 0.40-0.68%(P < 0.0001)。在由三位内分泌专家组成的小组对 60 个不同病例进行的定性评估中,AIDA 的建议大多被评为合理、精确。最后,与其他竞争方法相比,AIDA 能够考虑药物剂量的具体情况,因此具有一些优势,包括更符合实践偏好和临床指南,可提供实用但有效的选择、基于适应症的治疗和肾脏剂量。由于 AIDA 提供的药物剂量建议可改善 T2D 患者的个体治疗效果,因此可用于护理点的临床决策支持,尤其是在资源有限的环境中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


