Revealing the Chemical and Structural Complexity of Electrochemical Ion Exchange in Layered Oxide Materials
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Soft chemistry techniques, such as ion exchange, hold great potential for the development of battery electrode materials that cannot be stabilized via conventional equilibrium synthesis methods. Nevertheless, the intricate mechanisms governing ion exchange remain elusive. Herein, we investigate the evolution of the long-range and local structure, as well as the ion (de)intercalation mechanism during electrochemical Li-to-Na ion exchange initiated from an O3-type lithium-layered oxide cathode. The in situ-formed mixed-cation electrolyte leads to competitive intercalation of Li and Na ions. While Li ion intercalation predominates at the beginning of initial discharge, Na ion cointercalation into a different layer results in ion redistribution and phase separation, with the emergence of a P3–Na phase alongside an O3–Li phase. Further, this study spatially resolves the heterogeneous nature of electrochemical ion exchange reactions within individual particles and provides insights into the correlations between local Ni redox processes and phase separation. Overall, electrochemical ion exchange leads to a mixed-phase cathode and alters its reaction kinetics. Those findings have important implications for the development of new metastable materials for renewable energy devices and ion separation applications.
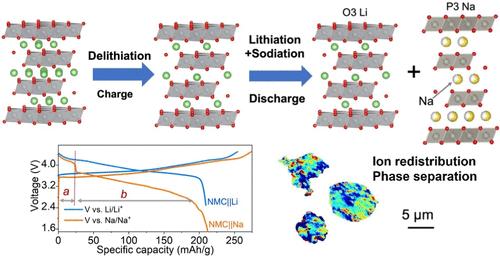
揭示层状氧化物材料中电化学离子交换的化学和结构复杂性
离子交换等软化学技术在开发无法通过传统平衡合成方法稳定的电池电极材料方面具有巨大潜力。然而,离子交换的复杂机制仍然难以捉摸。在此,我们研究了由 O3 型锂层氧化物正极引发的电化学锂-纳离子交换过程中长程和局部结构的演变,以及离子(脱)插层机制。原位形成的混合阳离子电解质导致了锂离子和钠离子的竞争性插层。锂离子插层在初始放电初期占主导地位,而 Na 离子共插层到不同的层会导致离子重新分布和相分离,出现 P3-Na 相和 O3-Li 相。此外,这项研究还从空间角度解析了单个颗粒内电化学离子交换反应的异质性,并深入分析了局部镍氧化还原过程与相分离之间的关联。总之,电化学离子交换会导致混相阴极并改变其反应动力学。这些发现对开发用于可再生能源设备和离子分离应用的新型可迁移材料具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


