Perovskite-Like Carbodiimides AB(NCN)3: Synthesis and Characterization of MnHf(NCN)3 and FeHf(NCN)3
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Two novel ternary air-stable transition-metal carbodiimides, MnHf(NCN)3 and FeHf(NCN)3, were synthesized via solid-state metathesis using either ZnNCN or Na2NCN as the carbodiimide source and the corresponding binary metal chlorides. These two phases are the first examples of transition-metal carbodiimides with an AB(NCN)3 composition, akin to ubiquitous ABO3 perovskite oxides. The crystal structure of MnHf(NCN)3 was determined and refined from powder X-ray diffraction (XRD) data in the non-centrosymmetric space group P6322 allowing for chirality, the assignment of which is supported by second-harmonic generation (SHG) measurements. FeHf(NCN)3 was found to crystallize isotypically, and the presence of iron(II) in a high spin state was confirmed by 57Fe Mößbauer spectroscopy. The structures are revealed to be NiAs-derived and can be described as a hexagonal stack of NCN2– anions with metal cations occupying 2/3 of the octahedral voids. Both IR spectroscopic measurements and DFT calculations agree that the NCN2– unit is a bent carbodiimide with C2v symmetry, necessary to account for the size difference present in such a vacancy-ordered structure. Magnetic studies reveal predominantly strong antiferromagnetic interactions but no long-range order between the paramagnetic Mn2+ centers, likely due to the dilution of Mn2+ over the octahedral sites or perhaps even due to some degree of magnetic frustration. The optical and electrochemical properties of MnHf(NCN)3 were then studied, revealing a wide band gap of 3.04 eV and p-type behavior.
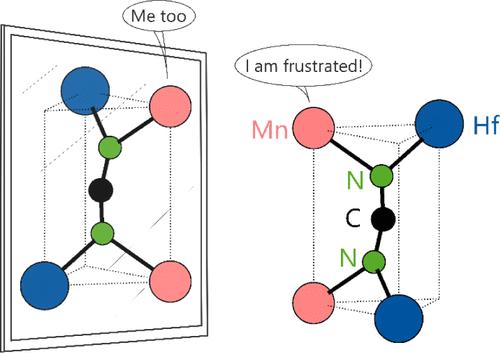
包晶类碳化二亚胺 AB(NCN)3:MnHf(NCN)3 和 FeHf(NCN)3 的合成与表征
以 ZnNCN 或 Na2NCN 作为碳二亚胺源,并使用相应的二元金属氯化物,通过固态偏析合成了两种新型三元空气稳定过渡金属碳二亚胺--MnHf(NCN)3 和 FeHf(NCN)3。这两种物相是具有 AB(NCN)3 成分的过渡金属碳二亚胺的首个实例,类似于无处不在的 ABO3 包晶氧化物。根据粉末 X 射线衍射(XRD)数据,确定并完善了 MnHf(NCN)3 的晶体结构,其非中心对称空间群为 P6322,允许存在手性,二次谐波发生(SHG)测量支持了手性的分配。研究发现,FeHf(NCN)3 具有同型结晶,57Fe Mößbauer 光谱证实了高自旋态铁(II)的存在。这些结构被揭示为源自 NiAs,可描述为 NCN2-阴离子的六方堆叠,金属阳离子占据了八面体空隙的 2/3。红外光谱测量和 DFT 计算都表明,NCN2- 单元是一种具有 C2v 对称性的弯曲碳二亚胺,这是解释这种空位有序结构中存在的尺寸差异所必需的。磁性研究显示,顺磁 Mn2+ 中心之间主要存在较强的反铁磁性相互作用,但没有长程有序性,这可能是由于八面体位点上的 Mn2+ 稀释,甚至可能是由于某种程度的磁挫折。随后研究了 MnHf(NCN)3 的光学和电化学特性,发现其具有 3.04 eV 的宽带隙和 p 型行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


