Cobalt-Catalyzed Asymmetric Migratory Nozaki–Hiyama–Kishi Coupling
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
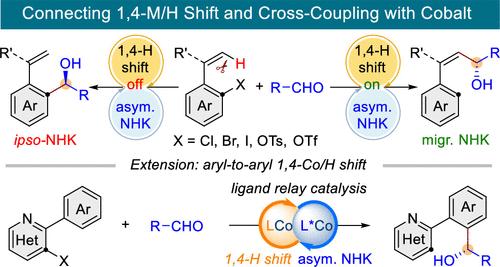
Selective functionalization of ubiquitous C–H bonds based on 1,n-metal migration provides an attractive and sustainable route to access complex molecules from readily available precursors. Herein, we report a Co-catalyzed asymmetric reductive migratory Nozaki–Hiyama–Kishi (NHK) coupling between two readily available electrophiles, aryl (pseudo)halides and aldehydes, via an unprecedented through-space aryl-to-alkenyl 1,4-cobalt/hydride shift. The judicious choice of ligands is crucial for selectivity, leading to either ipso- or migratory NHK products with exquisite control of regio-, E/Z-, and enantioselectivity. Enabled by a ligand relay catalytic strategy, this platform has been further extended to aryl-to-aryl asymmetric migratory NHK coupling. These high-value NHK adducts, including α-chiral allylic alcohols and benzyl alcohols, are readily convertible to a variety of useful synthons.
钴催化的不对称迁移野崎-希山-岸耦合
基于 1,n-金属迁移对无处不在的 C-H 键进行选择性官能化为从现成的前体中获得复杂分子提供了一条极具吸引力且可持续的途径。在此,我们报告了一种共催化的不对称还原迁移性野崎-希山-岸(NHK)偶联反应,该反应通过前所未有的通过空间的芳基到烯基的 1,4-钴/酐迁移,在芳基(假)卤化物和醛这两种容易获得的亲电体之间发生。配体的明智选择对选择性至关重要,可产生同源或迁移 NHK 产物,并对区域选择性、E/Z 选择性和对映体选择性进行精细控制。在配体中继催化策略的支持下,这一平台进一步扩展到了芳基对芳基不对称迁移 NHK 偶联。这些高价值的 NHK 加合物(包括 α-手性烯丙基醇和苄基醇)很容易转化为各种有用的合成物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


