Chemoenzymatic Labeling, Detection and Profiling of Core Fucosylation in Live Cells
IF 3.784
3区 化学
Q1 Chemistry
引用次数: 0
Abstract
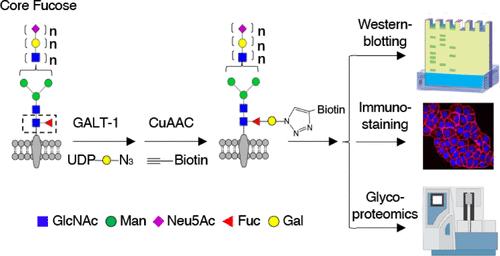
Core fucosylation, the attachment of an α-1,6-linked-fucose to the N-glycan core pentasaccharide, is an abundant protein modification that plays critical roles in various biological processes such as cell signaling, B cell development, antibody-dependent cellular cytotoxicity, and oncogenesis. However, the tools currently used to detect core fucosylation suffer from poor specificity, exhibiting cross-reactivity against all types of fucosylation. Herein we report the development of a new chemoenzymatic strategy for the rapid and selective detection of core fucosylated glycans. This approach employs a galactosyltransferase enzyme identified fromCaenorhabditis elegansthat specifically transfers an azido-appended galactose residue onto core fucose via a β-1,4 glycosidic linkage. We demonstrate that the approach exhibits superior specificity toward core fucose on a variety of complex N-glycans. The method enables detection of core fucosylated glycoproteins from complex cell lysates, as well as on live cell surfaces, and it can be integrated into a diagnostic platform to profile protein-specific core fucosylation levels. This chemoenzymatic labeling approach offers a new strategy for the identification of disease biomarkers and will allow researchers to further characterize the fundamental role of this important glycan in normal and disease physiology.
活细胞中核心岩藻糖基化的化学酶标记、检测和剖析
核心岩藻糖基化是指在 N-聚糖核心五糖上连接α-1,6-岩藻糖,它是一种丰富的蛋白质修饰,在细胞信号传导、B 细胞发育、抗体依赖性细胞毒性和肿瘤发生等多种生物过程中发挥着关键作用。然而,目前用于检测核心岩藻糖基化的工具特异性差,对所有类型的岩藻糖基化都有交叉反应。在此,我们报告了一种用于快速、选择性检测核心岩藻糖基化聚糖的新型化学酶法。这种方法采用了一种从大象猫科动物中发现的半乳糖基转移酶,它能通过β-1,4糖苷键将叠氮添加的半乳糖残基特异性地转移到核心岩藻糖上。我们证明了这种方法对各种复杂 N-聚糖上的核心岩藻糖具有超强的特异性。该方法可检测复杂细胞裂解液以及活细胞表面的核心岩藻糖基化糖蛋白,并可将其整合到诊断平台中,以分析蛋白质特异性核心岩藻糖基化水平。这种化学酶标记方法为疾病生物标记物的鉴定提供了一种新策略,并将使研究人员能够进一步确定这种重要聚糖在正常和疾病生理学中的基本作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Combinatorial Science
CHEMISTRY, APPLIED-CHEMISTRY, MEDICINAL
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
The Journal of Combinatorial Chemistry has been relaunched as ACS Combinatorial Science under the leadership of new Editor-in-Chief M.G. Finn of The Scripps Research Institute. The journal features an expanded scope and will build upon the legacy of the Journal of Combinatorial Chemistry, a highly cited leader in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


