Copper-Catalyzed Amination of Aryl Chlorides under Mild Reaction Conditions
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report a mild method for the copper-catalyzed amination of aryl chlorides. Key to the success of the method was the use of highly sterically encumbered N1,N2-diaryl diamine ligands which resist catalyst deactivation, allowing reactions to proceed at significantly lower temperatures and with a broader scope than current protocols. A sequence of highly chemoselective C–N and C–O cross-coupling reactions were demonstrated, and mechanistic studies indicate that oxidative addition of the Cu catalyst to the aryl chlorides is rate-limiting. We anticipate that the design principles disclosed herein will help motivate further advances in Cu-catalyzed transformations of aryl chlorides.
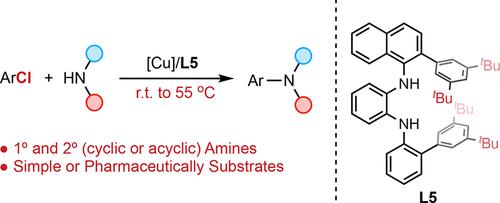
温和反应条件下铜催化的芳基氯胺化反应
我们报告了一种温和的铜催化芳基氯胺化方法。该方法成功的关键在于使用了高度立体包被的 N1,N2-二芳基二胺配体,这种配体可防止催化剂失活,从而使反应能够在明显更低的温度下进行,且反应范围比现有方案更广。一系列高化学选择性的 C-N 和 C-O 交叉偶联反应得到了证实,机理研究表明,铜催化剂与芳基氯化物的氧化加成是限速反应。我们预计,本文披露的设计原则将有助于推动铜催化芳基氯化物转化的进一步发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


