pH Affects the Spontaneous Formation of H2O2 at the Air–Water Interfaces
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
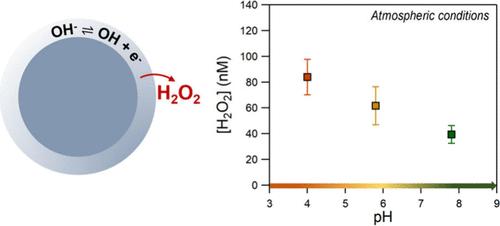
Recent studies have shown that the air–water interface of aqueous microdroplets is a source of OH radicals and hydrogen peroxide in the atmosphere. Several parameters such as droplet size, salt, and organic content have been suggested to play key roles in the formation of these oxidants. In this study, we focus on the effect of acidity on the spontaneous interfacial hydrogen peroxide formation of salt-containing droplets. Na2SO4, NaCl, and NaBr bulk solutions, at the range of pH 4 to 9.5, were nebulized, using ultra high-purity N2/O2 (80%/20%), and H2O2 was measured in the collected droplets. All of the experiments were performed in T = 292 ± 1 K and humidity levels of 90 ± 2%. For Na2SO4 and NaCl, the H2O2 concentration was increased by ∼40% under alkaline conditions, suggesting that OH– enriched environments promote its production. When CO2 was added in the ultrapure air, H2O2 was observed to be lower at higher pH. This suggests that dissolved CO2 can initiate reactions with OH radicals and electrons, impacting the interfacial H2O2 production. H2O2 formation in NaBr droplets did not display any dependence on the pH or the bath gas, showing that secondary reactions occur at the interface in the presence of Br–, which acts as an efficient interfacial source of electrons.
pH 值影响空气-水界面上 H2O2 的自发形成
最近的研究表明,水性微液滴的气水界面是大气中 OH 自由基和过氧化氢的来源。水滴大小、盐分和有机物含量等参数被认为在这些氧化剂的形成过程中起着关键作用。在本研究中,我们重点研究了酸度对含盐液滴自发形成界面过氧化氢的影响。使用超高纯度的 N2/O2(80%/20%)对 pH 值为 4 至 9.5 的 Na2SO4、NaCl 和 NaBr 体积溶液进行雾化,并在收集的液滴中测量 H2O2。所有实验均在温度 = 292 ± 1 K 和湿度为 90 ± 2% 的条件下进行。在碱性条件下,Na2SO4 和 NaCl 的 H2O2 浓度增加了 40%,这表明富含 OH 的环境促进了 H2O2 的产生。当在超纯空气中加入 CO2 时,观察到在较高的 pH 值下 H2O2 浓度较低。这表明溶解的 CO2 可与 OH 自由基和电子发生反应,从而影响界面 H2O2 的生成。NaBr 液滴中 H2O2 的形成与 pH 值或浴气没有任何关系,这表明在有 Br- 存在的情况下,界面上会发生二次反应,而 Br- 是有效的界面电子源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


