NY-ESO-1 antigen: A promising frontier in cancer immunotherapy
Abstract
Significant strides have been made in identifying tumour-associated antigens over the past decade, revealing unique epitopes crucial for targeted cancer therapy. Among these, the New York esophageal squamous cell carcinoma (NY-ESO-1) protein, a cancer/testis antigen, stands out. This protein is presented on the cell surface by major histocompatibility complex class I molecules and exhibits restricted expression in germline cells and various cancers, marking it as an immune-privileged site. Remarkably, NY-ESO-1 serves a dual role as both a tumour-associated antigen and its own adjuvant, implying a potential function as a damage-associated molecular pattern. It elicits strong humoural immune responses, with specific antibody frequencies significantly correlating with disease progression. These characteristics make NY-ESO-1 an appealing candidate for developing effective and specific immunotherapy, particularly for advanced stages of disease. In this review, we provide a comprehensive overview of NY-ESO-1 as an immunogenic tumour antigen. We then explore the diverse strategies for targeting NY-ESO-1, including cancer vaccination with peptides, proteins, DNA, mRNA, bacterial vectors, viral vectors, dendritic cells and artificial adjuvant vector cells, while considering the benefits and drawbacks of each strategy. Additionally, we offer an in-depth analysis of adoptive T-cell therapies, highlighting innovative techniques such as next-generation NY-ESO-1 T-cell products and the integration with lymph node-targeted vaccines to address challenges and enhance therapeutic efficacy. Overall, this comprehensive review sheds light on the evolving landscape of NY-ESO-1 targeting and its potential implications for cancer treatment, opening avenues for future tailored directions in NY-ESO-1-specific immunotherapy.
Highlights
-
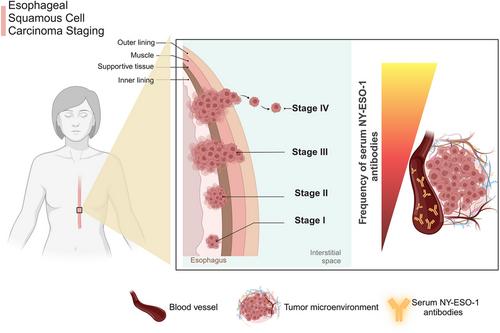
Endogenous immune response: NY-ESO-1 exhibited high immunogenicity, activating endogenous dendritic cells, T cells and B cells.
-
NY-ESO-1-based cancer vaccines: NY-ESO-1 vaccines using protein/peptide, RNA/DNA, microbial vectors and artificial adjuvant vector cells have shown promise in enhancing immune responses against tumours.
-
NY-ESO-1-specific T-cell receptor-engineered cells: NY-ESO-1-targeted T cells, along with ongoing innovations in engineered natural killer cells and other cell therapies, have improved the efficacy of immunotherapy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


