Kinetics and Equilibria Interconverting Aqueous Inorganic Chloramines: Errors and Corrections
IF 4.8
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
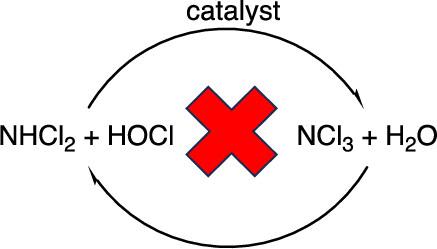
The three inorganic chloramines play central roles in aqueous chloramination processes. Mechanisms involving these species are required to obey the principle of detailed balancing, but very few published examples meet this requirement. There are at least 77 publications with chloramine mechanisms that violate the principle of detailed balancing. In this work, the violations are summarized, a set of reaction equilibrium constants and rate constants is proposed that should facilitate the development of acceptable mechanisms, and solutions to the published errors are proposed.
无机氯胺水溶液相互转化的动力学和平衡:错误与更正
三种无机氯胺在水氯化过程中发挥着核心作用。涉及这些物种的机理必须遵守详细平衡原则,但已发表的符合这一要求的例子却寥寥无几。至少有 77 篇出版物中的氯胺机理违反了详细平衡原则。在这项工作中,我们总结了这些违反原则的情况,提出了一套反应平衡常数和速率常数,这套常数应有助于建立可接受的机理,并提出了解决已发表错误的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


