Platelet margination dynamics in blood flow: The role of lift forces and red blood cells aggregation
IF 2.5
3区 物理与天体物理
Q2 PHYSICS, FLUIDS & PLASMAS
引用次数: 0
Abstract
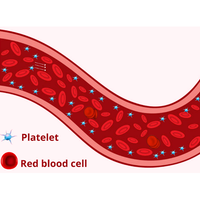
Homeostasis plays a critical role in maintaining the delicate balance between preventing excessive bleeding and enabling clot formation during injuries. One pivotal aspect of homeostasis involves the development of platelet clots. In this study, we analyze numerically the behavior of platelet margination as a function of the adhesion energy between red blood cells (RBCs), driven by the presence of plasma proteins. We examine scenarios encompassing both physiological conditions and pathological states, such as those seen in patients with diabetes. Employing a two-dimensional simulation, we utilize rigid particles and a vesicle model to simulate platelets and RBCs, respectively. We employ the lattice Boltzmann method to solve the underlying model equations. We first demonstrate that platelet margination is primarily determined by lift forces and is not notably affected by whether the cells undergo tank-treading (TT) or tumbling (TB) behavior, as often reported. Specifically, we unveil instances where cells exhibit TT or TB behavior, yet their platelet concentration profiles closely resemble each other. Furthermore, we present a striking result concerning the impact of RBC adhesion. In microcirculation the hematocrit is in the range . A moderate adhesion energy (falling within the physiological range) boosts platelet margination in microcirculation. However, this effect becomes small for larger hematocrit encountered in macrocirculation (e.g., ). This boost is more significant for a viscosity contrast (viscosity of cytoplasm over that the suspending fluid) equal to a known value for RBCs, as compared to the case without viscosity contrast. As we increase the adhesion energy (the pathological range), a noteworthy decline in platelet margination is found, albeit that for some flow strength the platelet margination reaches a minimum and increases again at higher adhesion energy. These results can be attributed to a combination of lift generated by the bounding walls and the formation of RBC clusters. Notably, our study sheds light on a critical consequence of excessive adhesion, typically observed in pathological conditions like diabetes mellitus.
血流中的血小板边缘动力学:升力和红细胞聚集的作用
平衡状态在维持防止过度出血和受伤时血凝块形成之间的微妙平衡方面起着至关重要的作用。平衡的一个关键方面涉及血小板凝块的形成。在本研究中,我们通过数值分析了血小板边缘化的行为,它是红细胞(RBC)之间粘附能量的函数,由血浆蛋白的存在驱动。我们研究了包括生理条件和病理状态(如糖尿病患者的病理状态)在内的各种情况。通过二维模拟,我们利用刚性粒子和囊泡模型分别模拟血小板和红细胞。我们采用晶格玻尔兹曼法求解基础模型方程。我们首先证明了血小板边缘化主要是由升力决定的,并不像通常报道的那样受细胞是否发生坦克踏步(TT)或翻滚(TB)行为的显著影响。具体来说,我们揭示了细胞表现出 TT 或 TB 行为,但它们的血小板浓度分布却非常相似的情况。此外,我们还提出了一个关于红细胞粘附影响的惊人结果。在微循环中,血细胞比容在 5-20% 之间。适度的粘附能量(在生理范围内)会促进微循环中血小板的边缘化。然而,在大循环中遇到较大的血细胞比容(如 40%)时,这种作用就变得很小。与没有粘度对比的情况相比,当粘度对比(细胞质的粘度大于悬浮液的粘度)等于已知的红细胞值时,这种促进作用更为明显。随着粘附能量(病理范围)的增加,我们发现血小板边缘化显著下降,尽管在某些流动强度下,血小板边缘化达到最小值,但在粘附能量较高时,血小板边缘化又会增加。这些结果可归因于结合壁产生的升力和红细胞簇的形成。值得注意的是,我们的研究揭示了过度粘附的一个重要后果,这通常在糖尿病等病理情况下可以观察到。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Physical Review Fluids
Chemical Engineering-Fluid Flow and Transfer Processes
CiteScore
5.10
自引率
11.10%
发文量
488
期刊介绍:
Physical Review Fluids is APS’s newest online-only journal dedicated to publishing innovative research that will significantly advance the fundamental understanding of fluid dynamics. Physical Review Fluids expands the scope of the APS journals to include additional areas of fluid dynamics research, complements the existing Physical Review collection, and maintains the same quality and reputation that authors and subscribers expect from APS. The journal is published with the endorsement of the APS Division of Fluid Dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


