ALDH1A3 is the switch that determines the balance of ALDH+ and CD24−CD44+ cancer stem cells, EMT-MET, and glucose metabolism in breast cancer
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Plasticity is an inherent feature of cancer stem cells (CSCs) and regulates the balance of key processes required at different stages of breast cancer progression, including epithelial-to-mesenchymal transition (EMT) versus mesenchymal-to-epithelial transition (MET), and glycolysis versus oxidative phosphorylation. Understanding the key factors that regulate the switch between these processes could lead to novel therapeutic strategies that limit tumor progression. We found that aldehyde dehydrogenase 1A3 (ALDH1A3) regulates these cancer-promoting processes and the abundance of the two distinct breast CSC populations defined by high ALDH activity and CD24−CD44+ cell surface expression. While ALDH1A3 increases ALDH+ breast cancer cells, it inversely suppresses the CD24−CD44+ population by retinoic acid signaling-mediated gene expression changes. This switch in CSC populations induced by ALDH1A3 was paired with decreased migration but increased invasion and an intermediate EMT phenotype. We also demonstrate that ALDH1A3 increases oxidative phosphorylation and decreases glycolysis and reactive oxygen species (ROS). The effects of ALDH1A3 reduction were countered with the glycolysis inhibitor 2-deoxy-D-glucose (2DG). In cell culture and tumor xenograft models, 2DG suppresses the increase in the CD24−CD44+ population and ROS induced by ALDH1A3 knockdown. Combined inhibition of ALDH1A3 and glycolysis best reduces breast tumor growth and tumor-initiating cells, suggesting that the combination of targeting ALDH1A3 and glycolysis has therapeutic potential for limiting CSCs and tumor progression. Together, these findings identify ALDH1A3 as a key regulator of processes required for breast cancer progression and depletion of ALDH1A3 makes breast cancer cells more susceptible to glycolysis inhibition.
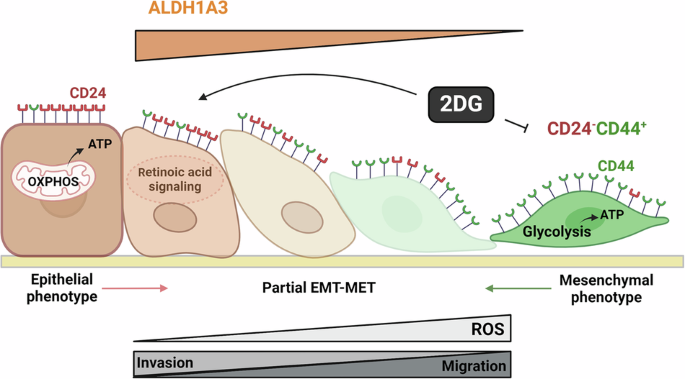
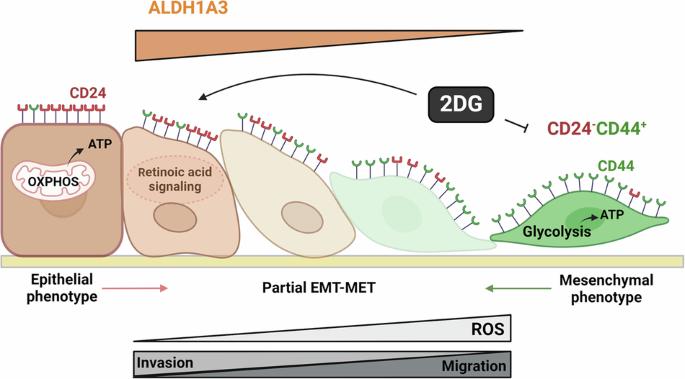
ALDH1A3 是决定乳腺癌中 ALDH+ 和 CD24-CD44+ 癌症干细胞、EMT-MET 以及葡萄糖代谢平衡的开关
可塑性是癌症干细胞(CSCs)的固有特征,它调节着乳腺癌进展不同阶段所需的关键过程的平衡,包括上皮到间质转化(EMT)与间质到上皮转化(MET),以及糖酵解与氧化磷酸化。了解调控这些过程之间转换的关键因素,可以找到限制肿瘤进展的新型治疗策略。我们发现,醛脱氢酶1A3(ALDH1A3)调控着这些促癌过程以及由高ALDH活性和CD24-CD44+细胞表面表达所定义的两种不同乳腺癌细胞间充质干细胞群的丰度。ALDH1A3 在增加 ALDH+ 乳腺癌细胞的同时,通过维甲酸信号介导的基因表达变化,反向抑制了 CD24-CD44+ 群体。ALDH1A3 诱导的 CSC 群体的这种变化与迁移减少、侵袭增加和中间 EMT 表型相匹配。我们还证明,ALDH1A3 增加了氧化磷酸化,减少了糖酵解和活性氧(ROS)。糖酵解抑制剂 2-脱氧-D-葡萄糖(2DG)可对抗 ALDH1A3 减少的影响。在细胞培养和肿瘤异种移植模型中,2DG 可抑制 ALDH1A3 基因敲除诱导的 CD24-CD44+ 群体和 ROS 的增加。联合抑制 ALDH1A3 和糖酵解能最大程度地减少乳腺肿瘤的生长和肿瘤诱发细胞,这表明联合靶向 ALDH1A3 和糖酵解具有限制 CSCs 和肿瘤进展的治疗潜力。总之,这些发现确定了ALDH1A3是乳腺癌进展过程所需的一个关键调节因子,而ALDH1A3的耗竭会使乳腺癌细胞更容易受到糖酵解抑制的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


