New Heteroligand Octahedral Cluster Complexes of Molybdenum and Tungsten
IF 1.2
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The (Bu4N)2[{Mo6I8}(CN)5(OEt)]·2H2O and (Bu4N)2[{W6I8}(CN)4(OEt)2]·H2O·EtOH cluster complexes are prepared by the reaction of (Bu4N)2[{M6I8}I6] (M = Mo, W) with NaCN in ethanol and subsequent recrystallization from an aqueous ethanol solution. The structure of both compounds is determined by XRD. The external ligand environment of the anions contains CN– and OEt– ligands. The behavior of the complexes in an aqueous solution is studied by electron spectroscopy.
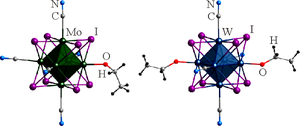

钼和钨的新型异配位体八面体簇合物
摘要 (Bu4N)2[{Mo6I8}(CN)5(OEt)]-2H2O和(Bu4N)2[{W6I8}(CN)4(OEt)2]-H2O-EtOH簇合物是由(Bu4N)2[{M6I8}I6](M = Mo,W)与 NaCN 在乙醇中反应,然后从乙醇水溶液中重结晶制备的。这两种化合物的结构都是通过 XRD 确定的。阴离子的外部配体环境包含 CN- 和 OEt- 配体。电子光谱法研究了这些配合物在水溶液中的行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


